Which of these enters the citric acid cycle?
-G3P
-glucose
-acetyl CoA
-NADH + H+
-pyruvate
Acetyl CoA
In the citric acid cycle, ATP molecules are produced by _____.
cellular respiration
photosynthesis
substrate-level
phosphorylation
photophosphorylation
oxidative phosphorylation
-substrate-level phosphorylation
Which of these is NOT a product of the citric acid cycle?
ATP
FADH2
CO2
NADH + H+
acetyl CoA
-Acetyl CoA
In the oxidation of pyruvate to acetyl CoA, one carbon atom is released as CO2. However, the oxidation of the remaining two carbon atoms—in acetate—to CO2 requires a complex, eight-step pathway—the citric acid cycle. Consider four possible explanations for why the last two carbons in acetate are converted to CO2 in a complex cyclic pathway rather than through a simple, linear reaction.
Use your knowledge of the first three stages of cellular respiration to determine which explanation is correct.
-More ATP is produced per CO2 released in cyclic processes than
in linear processes.
-It is easier to remove electrons and
produce CO2 from compounds with three or more carbon atoms than from
a two-carbon compound such as acetyl CoA.
-Redox reactions that
simultaneously produce CO2 and NADH occur only in cyclic
processes.
-Cyclic processes, such as the citric acid cycle,
require a different mechanism of ATP synthesis than linear processes,
such as glycolysis.
-It is easier to remove electrons and produce CO2 from compounds with three or more carbon atoms than from a two-carbon compound such as acetyl CoA
Although it is possible to oxidize the two-carbon acetyl group of acetyl CoA to two molecules of CO2, it is much more difficult than adding the acetyl group to a four-carbon acid to form a six-carbon acid (citrate). Citrate can then be oxidized sequentially to release two molecules of CO2.
Which molecule is metabolized in a cell to produce energy for performing work?
Phosphate
ATP
ADP
Glucose
-Glucose
Glucose is used to produce high-energy ATP in a cell.
True or false? The potential energy in an ATP molecule is derived mainly from its three phosphate groups.
True
The three phosphate groups in an ATP molecule carry negative charges that strongly repel each other and give ATP a large amount of potential energy.
Which process is not part of the cellular respiration pathway that produces large amounts of ATP in a cell?
Glycolysis
Krebs cycle
Electron transport chain
Fermentation
Fermentation
Fermentation is an alternate pathway used when oxygen levels are low.
Which step of the cellular respiration pathway can take place in the absence of oxygen?
Krebs cycle
Electron transport chain
Glycolysis
Fermentation
Glycolysis
Glycolysis can take place in the absence of oxygen; its product, pyruvate, enters the cellular respiration pathway or undergoes fermentation depending on the availability of oxygen.
Into which molecule are all the carbon atoms in glucose ultimately incorporated during cellular respiration?
NADH
Carbon dioxide
ATP
Water
Carbon Dioxide
All of the carbon atoms in glucose are incorporated into carbon dioxide: Two molecules are formed as pyruvate is converted to acetyl CoA, and four molecules are formed during the Krebs cycle.
Which of the following statements about the electron transport chain is true?
The electron transport chain is the first step in cellular
respiration.
NADH and FADH2 donate their electrons to the
chain.
Water is the last electron acceptor.
Electrons gain
energy as they move down the chain.
NADH and FADH2 donate their electrons to the chain
The electrons lose energy as they move down the chain, and this energy is used to create a proton gradient that drives the synthesis of ATP.
Which stage of glucose metabolism produces the most ATP?
Krebs cycle
Electron transport and
chemiosmosis
Fermentation of pyruvate to lactate
Glycolysis
Electron transport and chemiosmosis
Electron transport and chemiosmosis (oxidative phosphorylation) can yield around 26 molecules of ATP.
True or false? The reactions that generate the largest amounts of ATP during cellular respiration take place in the mitochondria.
True
False
TRUE
Glycolysis takes place in the cytoplasm, whereas the Krebs cycle and the electron transport chain, which generate the largest amounts of ATP during cellular respiration, take place in the mitochondria.
In mitochondrial electron transport, what is the direct role of O2?
-to function as the final electron acceptor in the electron
transport chain
-to provide the driving force for the production
of a proton gradient
-to oxidize NADH and FADH2 from glycolysis,
acetyl CoA formation, and the citric acid cycle
-to provide the
driving force for the synthesis of ATP from ADP and Pi
To function as the final electron acceptor in the electron transport chain
The only place that O2 participates in cellular respiration is at the end of the electron transport chain, as the final electron acceptor. Oxygen's high affinity for electrons ensures its success in this role. Its contributions to driving electron transport, forming a proton gradient, and synthesizing ATP are all indirect effects of its role as the terminal electron acceptor.
How would anaerobic conditions (when no O2 is present) affect the rate of electron transport and ATP production during oxidative phosphorylation? (Note that you should not consider the effect on ATP synthesis in glycolysis or the citric acid cycle.)
Neither electron transport nor ATP synthesis would be
affected.
Electron transport would stop but ATP synthesis would
be unaffected.
Electron transport would be unaffected but ATP
synthesis would stop.
Both electron transport and ATP synthesis
would stop.
-Both electron transport and ATP synthesis would stop
Oxygen plays an essential role in cellular respiration because it is the final electron acceptor for the entire process. Without O2, mitochondria are unable to oxidize the NADH and FADH2 produced in the first three steps of cellular respiration, and thus cannot make any ATP via oxidative phosphorylation. In addition, without O2 the mitochondria cannot oxidize the NADH and FADH2 back to NAD+ and FAD, which are needed as inputs to the first three stages of cellular respiration.
NADH and FADH2 are both electron carriers that donate their electrons to the electron transport chain. The electrons ultimately reduce O2 to water in the final step of electron transport. However, the amount of ATP made by electrons from an NADH molecule is greater than the amount made by electrons from an FADH2 molecule.
Which statement best explains why more ATP is made per molecule of NADH than per molecule of FADH2?
-Fewer protons are pumped across the inner mitochondrial membrane
when FADH2 is the electron donor than when NADH is the electron
donor.
-It takes more energy to make ATP from ADP and Pi using
FADH2 than using NADH.
-FADH2 is made only in the citric acid
cycle while NADH is made in glycolysis, acetyl CoA formation, and the
citric acid cycle.
-The H+ gradient made from electron transport
using NADH is located in a different part of the mitochondrion than
the H+ gradient made using FADH2.
-There is more NADH than FADH2
made for every glucose that enters cellular respiration.
-Fewer protons are pumped across the inner mitochondrial membrane when FADH2 is the electron donor than when NADH is the electron donor.
Electrons derived from the oxidation of FADH2 enter the electron transport chain at Complex II, farther down the chain than electrons from NADH (which enter at Complex I). This results in fewer H+ ions being pumped across the membrane for FADH2 compared to NADH, as this diagram shows. Thus, more ATP can be produced per NADH than FADH2.
How many NADH are produced by glycolysis?
2
3
5
1
4
2
Two NADH molecules are produced by glycolysis.
In glycolysis, ATP molecules are produced by _____.
photosynthesis
photophosphorylation
oxidative
phosphorylation
substrate-level phosphorylation
cellular respiration
Substrate-level phosphorylation
A phosphate group is transferred from glyceraldehyde phosphate to ADP.
Which of these is NOT a product of glycolysis?
NADH
pyruvate
FADH2
ATP
FADH2
FADH2 is a product of the citric acid cycle.
In glycolysis, what starts the process of glucose oxidation?
NADPH
hexokinase
ADP
ATP
FADH2
-ATP
Some ATP energy is used to start the process of glucose oxidation.
In glycolysis there is a net gain of _____ ATP.
2
1
3
4
5
-2
It takes 2 ATP to produce 4 ATP.
When a compound donates (loses) electrons, that compound becomes _________. Such a compound is often referred to as an electron donor.
Oxidized
2. When a compound accepts (gains) electrons, that compound becomes __________. Such a compound is often referred to as an electron acceptor.
Reduced
3. In glycolysis, the carbon-containing compound that functions as the electron donor is ______________.
-Glucose
Once the electron donor in glycolysis gives up its electrons, it is oxidized to a compound called __________.
-Pyruvate
5. ____________ is the compound that functions as the electron acceptor in glycolysis.
-NAD+
6. The reduced form of the electron acceptor in glycolysis is ___________.
-NADH
Among the products of glycolysis, which compounds contain energy that can be used by other biological reactions?
CO2 only
ATP only
pyruvate and ATP only
ATP and NADH
only
pyruvate, ATP, and NADH
NADH only
O2 only
-pyruvate, ATP, NADH
ATP is the main product of cellular respiration that contains energy that can be used by other cellular processes. Some ATP is made in glycolysis. In addition, the NADH and pyruvate produced in glycolysis are used in subsequent steps of cellular respiration to make even more ATP.
Sort the statements into the appropriate bin depending on whether or not they correctly describe some aspect of substrate-level phosphorylation in glycolysis.
- An enzyme is required in order for the reaction to occur.
- One of the substrates is a molecule derived from the breakdown of glucose.
- A bond must be broken between an organic molecule and phosphate before ATP can form.
- The enzymes involved in ATP synthesis must be attached to a membrane to produce ATP.
- The phosphate group added to ADP to make ATP comes from free inorganic phosphate ions
CORRECT:
-An enzyme is required in order for the reaction to occur
-One of the substrates is a molecule derived from the breakdown of glucose
-A bond must be broken between an organic molecule and a phosphate before ATP can form
INCORRECT:
-The enzymes involved in ATP synthesis must be attached to a membrane to produce ATP.
-The phosphate group added to ADP to make ATP comes from free inorganic phosphate ions
In muscle cells, fermentation produces _____.
carbon dioxide, ethanol, NADH, and ATP
lactate and
NAD+
lactate, NADH, and ATP
pyruvate
carbon dioxide,
ethanol, and NAD+
lactate and NAD+
These are the products of fermentation as it occurs in muscle cells.
In fermentation _____ is reduced and _____ is oxidized.
NADH ... lactate
pyruvate ... NADH
lactate ...
NADH
NAD+ ... pyruvate
lactate ... ethanol
-pyruvate......NADH
The pyruvate from glycolysis is reduced to either lactate or ethanol, and NADH is oxidized to NAD+.
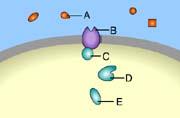
Part A
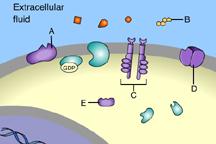
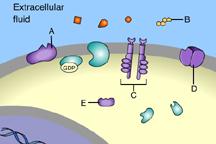
Which of these is a receptor molecule?
C
A
E
B
D
B
This is a receptor molecule.
A signal transduction pathway is initiated when a _____ binds to a receptor.
G protein
signal molecule
tyrosine kinase
cyclic AMP
calmodulin
Signal Molecule
The binding of a signal molecule to a receptor initiates a signal transduction pathway.
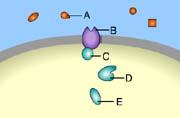
Which of these is a signal molecule?
A
E
B
C
D
.
A
This is a signal molecule
A signal molecule is also known as a(n) _____.
key
initiator
ligand
receptor
protein
Ligand
A ligand is a signal molecule.
Which of these is the second of the three stages of cell signaling?
gene activation
reception
binding of a neurotransmitter
to a plasma membrane receptor
transduction
cell response
Transduction
Transduction is the second
of the three stages of cell signaling.
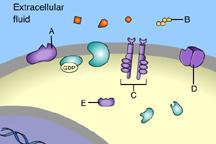
Which of these receptors is NOT a membrane receptor?
A
E
D
B
C
E
This receptor is not associated with the plasma membrane.
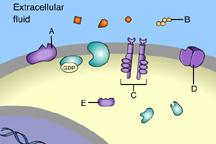
Which of these is a G-protein-linked receptor?
E
B
D
C
A
A
This is a G-protein-linked receptor.
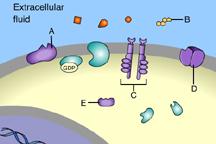
Which of these is a receptor tyrosine kinase?
C
A
E
B
D
C
This is a receptor tyrosine kinase.
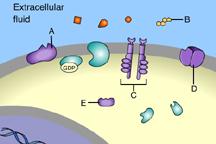
Which of these is an ion-channel receptor?
D
A
B
E
C
D
This receptor does form a channel.
The binding of signal molecules to _____ results in the phosphorylation of tyrosines.
A
B
E
D
C
C
The binding of signal molecules to tyrosine-kinase receptors activates tyrosine-kinase enzymes, which phosphorylate tyrosines.
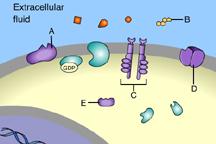
Which of these receptor molecules would allow Na+ to flow into the cell?
A
E
D
B
C
D
This is an ion-channel receptor.
Which of these extracellular signal molecules could diffuse through a plasma membrane and bind to an intracellular receptor?
glucose
estrogen
cellulose
starch
glycerol
.
Estrogen
Nonpolar molecules can diffuse through the plasma membrane and bind to intracellular receptors
A(n) _____ is an example of a signal molecule that can bind to an intracellular receptor and thereby cause a gene to be turned on or off.
carbohydrate
ion
protein
nucleic acid
steroid
Steroid
Steroids bind to intracellular receptors, which can then bind to, and regulate, the expression of genes.
_____ is a signal molecule that binds to an intracellular receptor
D
B
E
A
C
D
Steroids are nonpolar and can diffuse through the plasma membrane
Thyroid hormones bind to _____ receptors.
steroid
plasma membrane ion-channel
intracellular
G-protein-linked
tyrosine-kinase
Intracellular
Thyroid hormones are able to pass through the plasma membrane.
Which of these acts as a second messenger?
D
C
A
B
E
D
This is a second messenger.
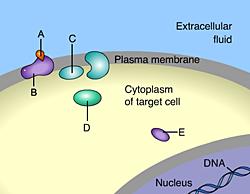
Which of these is responsible for initiating a signal transduction pathway?A
B
C
D
E
A
This is a signal molecule. The attachment of a signal molecule to a plasma membrane receptor initiates a signal transduction pathway.
What role does a transcription factor play in a signal transduction pathway?
-By binding to a plasma membrane receptor it initiates a
cascade.
-It relays a signal from the cytoplasm to the plasma
membrane.
-It activates relay proteins.
-By binding to DNA
it triggers the transcription of a specific gene.
-It is a plasma
membrane protein that binds signal molecules.
This is the
function of a transcription factor.
By binding to DNA it triggers the Transcription of a specific gene
This is the function of a transcription factor.
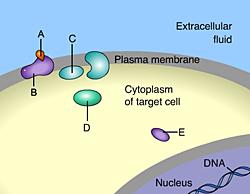
Which of these is a membrane receptor?
A
B
C
D
E
B
This is a receptor molecule.
A signal transduction pathway is initiated when a _____ binds to a receptor.
G protein
tyrosine kinase
calmodulin
signal
molecule
cyclic AMP
Signal Molecule
The binding of a signal molecule to a receptor initiates a signal trane
Which of these acts as a second messenger?
protein kinase
G-protein-linked receptor
G
protein
adenylyl kinase
cyclic AMP
Cyclic AMP
Cyclic AMP can act as second messengers.
Calcium ions that act as second messengers are stored in _____.
endoplasmic reticula
mitochondria
lysosomes
chloroplasts
peroxisomes
Endoplasmic Reticula
The ER stores calcium ions.
_____ catalyzes the production of _____, which then opens an ion channel that releases _____ into the cell's cytoplasm.
Adenylyl cyclase ... IP3 .... Ca2+
Phospholipase C ... cyclic
AMP ... Ca2+
Adenylyl cyclase ... cyclic AMP ...
Ca2+
Phospholipase C ... IP3 .... Ca2+
Protein kinase ...
PIP2 ... Na+
Phospholipase C ... IP3 .... Ca2+
Phospholipase C cleaves IP3 from a membrane protein, and IP3 then binds to a calcium channel on the ER.
A protein kinase activating many other protein kinases is an example of _____.
deactivation
mutualism
sensitization
a cellular response
amplification
Amplification
By activating many other molecules the initial signal is amplified.
The cleavage of glycogen by glycogen phosphorylase releases _____.
glucose-1-phosphate
nothing: glycogen phosphorylase cannot
cleave glycogen
cellulose
galactose-1-phosphate
fructose-1-phosphate
Glucose-1 phosphate
Glycogen is a polysaccharide composed of glucose monomers.
Epinephrine acts as a signal molecule that attaches to _____ proteins.
ion-channel receptor
G-protein-linked
receptor
intracellular receptor
receptor tyrosine
kinase
nuclear receptor
G-protein-linked receptor
Epinephrine acts via G-protein-linked receptors.
Which of these is activated by calcium ions?
G protein
calmodulin
IP3
PIP2
adenylyl cyclase
Calmodulin
Calmodulin is a calcium-binding protein.
Which of these is NOT correct?
Ion channels are found on both the plasma membrane and the
endoplasmic reticulum.
Cyclic AMP binds to
calmodulin.
Phospholipase C catalyzes the formation of
IP3.
Tyrosine-kinase receptors consist of two polypeptides that
join when activated by a signal molecule.
Kinases are enzymes
that phosphorylate other molecules.
Cyclic AMP binds to calmodulin
Calcium binds to calmodulin.
A toxin that inhibits the production of GTP would interfere with the function of a signal transduction pathway that is initiated by the binding of a signal molecule to _____ receptors.
intracellular
steroid
G-protein-linked
ion-channel
receptor
tyrosine kinase
G-Protein Linked
GTP activates G proteins.
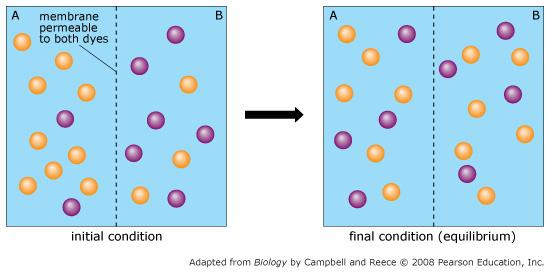
Orange dye moves independently of purple dye?
Always
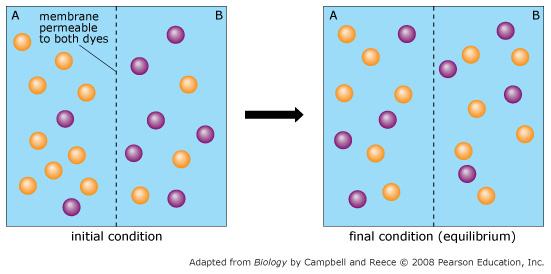
Concentration gradients exist that drive diffusion both dyes
Only before equilibrium is reached
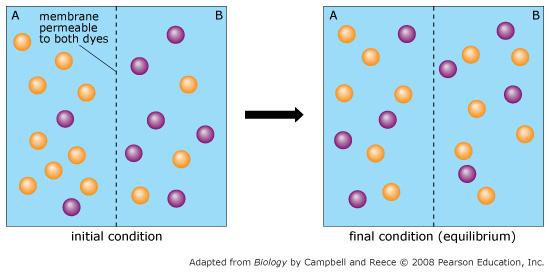
There is a net movement of orange dye from side A to B
Only before equilibrium is reached
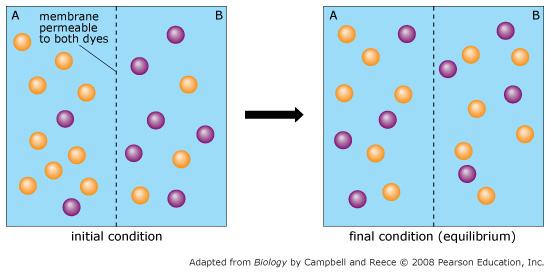
Purple dye moves only from side B to side A
Never
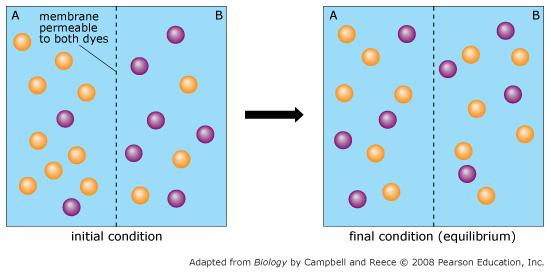
There is no movement of purple dye
Only at equilibrium
Some solutes are able to pass directly through the lipid bilayer of a
plasma membrane, whereas other solutes require a transport protein or
other mechanism to cross between the inside and the outside of a cell.
The fact that the plasma membrane is permeable to some solutes but not
others is what is referred to as selective permeability.
Which of
the following molecules can cross the lipid bilayer of a membrane
directly, without a transport protein or other mechanism? Select all
that apply.
lipids
oxygen
ions
water
proteins
sucrose
carbon dioxide
Lipids, Oxygen, Water, Carbon Dioxide
Some solutes pass readily through the lipid bilayer of a
cell membrane, whereas others pass through much more slowly, or
not at all.
Small nonpolar
(hydrophobic) molecules, such as dissolved gases (O2, CO2, N2)
and small lipids, can pass directly through the membrane. They do
so by interacting directly with the hydrophobic interior of the
lipid bilayer.
Very small
polar molecules such as water and glycerol can pass directly
through the membrane, but much more slowly than small nonpolar
molecules. The mechanism that permits small polar molecules to
cross the hydrophobic interior of the lipid bilayer is not
completely understood, but it must involve the molecules
squeezing between the hydrophobic tails of the lipids that make up
the bilayer.
Polar molecules
such as glucose and sucrose have very limited
permeability.
Large molecules
such as proteins cannot pass through the lipid
bilayer.
Ions and charged
molecules of any size are essentially impermeable to the lipid
bilayer because they are much more soluble in water than in the
interior of the membrane.
The majority of solutes that diffuse across the plasma membrane
cannot move directly through the lipid bilayer. The passive movement
of such solutes (down their concentration gradients without the input
of cellular energy) requires the presence of specific transport
proteins, either channels or carrier proteins. Diffusion through a
transport protein in the plasma membrane is called facilitated
diffusion.
Diagram showing facilitated diffusion across the
plasma membrane. A channel protein embedded in the membrane allows
yellow balls to travel through a channel from the outside of the cell
to the inside. A carrier protein embedded in the membrane undergoes a
shape change allowing red balls to travel from the outside of the cell
to the inside.
Sort the phrases into the appropriate bins
depending on whether they are true only for channels, true only for
carrier proteins, or true for both channels and carriers.
-
- provide a continuous path across the membrane
- allow water molecules and small ions to flow quickly across the membrane
- undergo a change in shape to transport solutes across the membrane
-
- transport primarily small polar organic molecules
- are integral membrane proteins
-
- transport solutes down a concentration or electrochemical gradient
- provide a hydrophilic path across the membrane
ONLY CHANNELS
- provide a continuous path across the membrane
- allow water molecules and small ions to flow quickly across the membrane
ONLY CARRIERS
- undergo a change in shape to transport solutes across the membrane
- transport primarily small polar organic molecules
BOTH CHANNELS AND CARRIERS
- are integral membrane proteins
- transport solutes down a concentration or electrochemical gradient
- provide a hydrophilic path across the membrane
Because ions carry a charge (positive or negative), their transport
across a membrane is governed not only by concentration gradients
across the membrane but also by differences in charge across the
membrane (also referred to as membrane potential). Together, the
concentration (chemical) gradient and the charge difference
(electrical gradient) across the plasma membrane make up the
electrochemical gradient.
Consider the plasma membrane of an
animal cell that contains a sodium-potassium pump as well as two
non-gated (always open) ion channels: a Na+ channel and a K+ channel.
The effect of the sodium-potassium pump on the concentrations of Na+
and K+ as well as the distribution of charge across the plasma
membrane is indicated in the figure below.
Which of the
following statements correctly describe(s) the driving forces for
diffusion of Na+ and K+ ions through their respective channels?
Select all that apply.
1. The diffusion of Na+ ions into the cell is facilitated by the
Na+ concentration gradient across the plasma membrane.
2. The
diffusion of Na+ ions into the cell is impeded by the electrical
gradient across the plasma membrane.
3.The diffusion of K+ ions
out of the cell is impeded by the K+ concentration gradient across the
plasma membrane.
4.The diffusion of K+ ions out of the cell is
impeded by the electrical gradient across the plasma membrane.
5.
The electrochemical gradient is larger for Na+ than for K+.
- The diffusion of Na+ ions into the cell is facilitated by the Na+ concentration gradient across the plasma membrane.
- The diffusion of K+ ions out of the cell impeded by the electrical gradient across the plasma membrane
- The electrochemical gradient is larger Na+ than for K+
The concentration gradient of K+ ions across the
membrane (higher K+ concentration inside) facilitates the
diffusion of K+ out of the cell. However, the electrical gradient
across the membrane (excess positive charge outside) impedes the
diffusion of K+ out of the cell.
The electrochemical gradient for an ion is the sum of the
concentration (chemical) gradient and the electrical gradient
(charge difference) across the membrane. For Na+ ions, diffusion
through the Na+ channel is driven by both the concentration
gradient and the electrical gradient. But for K+ ions, the
electrical gradient opposes the concentration gradient. Therefore,
the electrochemical gradient for Na+ is greater than the
electrochemical gradient for K+.
Which of the following statements is TRUE with regard to this animation?
Potassium ions are transported down their concentration
gradient.
The cell is not expending energy.
Both sodium and
potassium ions are transported against their concentration
gradients.
The cell does not expend ATP.
Sodium ions are
transported down their concentration gradient.
Both sodium and potassium ions are transported against their concentration gradients
Both ions are transported from where their concentration is low to where their concentration is high, and the cell expends energy in the form of ATP to do it.
Sort the phrases into the appropriate bins depending on whether they describe exocytosis, endocytosis, or both.
- requires fusion of vesicles with the plasma membrane
- secretes large molecules out of the cell
- increases the surface area of the plasma membrane
- forms vesicles from inward folding of the plasma membrane
- decreases the surface area of the plasma membrane
- requires cellular energy
- transported substances never physically cross the plasma membrane
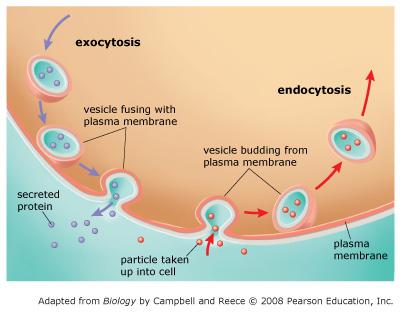
EXOCYTOSIS
- requires fusion of vesicles with the plasma membrane
- secretes large molecules out of the cell
- increases the surface area of the plasma membrane
ENDOCYTOSIS
- forms vesicles from inward folding of the plasma membrane
- decreases the surface area of the plasma membrane
BOTH
- requires cellular energy
- transported substances never physically cross the plasma membrane
In exocytosis, substances are transported to the plasma membrane in vesicles derived from the endomembrane system. These vesicles fuse with the plasma membrane, releasing the enclosed substances outside the cell.
In endocytosis, substances are taken into the cell by folding in of the plasma membrane and pinching off of the membrane to form a vesicle. Notice that in both exocytosis and endocytosis, the transported substances never actually cross the plasma membrane as they leave or enter the cell.
Endocytosis moves materials _____ a cell via _____.
into ... membranous vesicles
out of ... diffusion
into
... facilitated diffusion
out of ... membranous
vesicles
into ... a transport protein
Into...... membranous vesicles
The prefix "endo-" means "inward."
You can recognize the process of pinocytosis when _____.
Click
to launch animation
the cell is engulfing a large particle
the cell is engulfing
extracellular fluid
a receptor protein is involved
The cell is engulfing extracellular fluid
Pinocytosis is "cell drinking."
A white blood cell engulfing a bacterium is an example of _____.
exocytosis
receptor-mediated endocytosis
facilitated diffusion
pinocytosis
phagocytosis
Phagocytosis
Phagocytosis occurs when a cell engulfs a large particle.
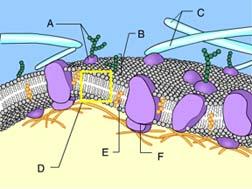
What is the function of Structure E?
detection of environmental change
structural support of the
cell
transport across the plasma membrane
stabilization of
the phospholipids
cell-cell communication
Stabilization of the phospholipids
Cholesterol helps to stabilize the structure of the plasma membrane.
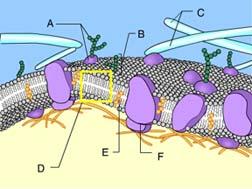
Identify Structure D.
protein
glycoprotein
extracellular
matrix
phospholipid bilayer of membrane
cholesterol
Phospholipid bilayer of membrane
Phospholipids can be recognized by the presence of a head and two tails.
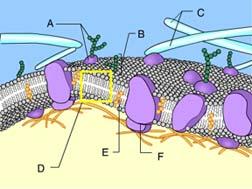
Identify Structure A.
protein
phospholipid
cholesterol
extracellular matrix
glycoprotein
Glycoprotein
Structure A is composed of both a carbohydrate and a protein.
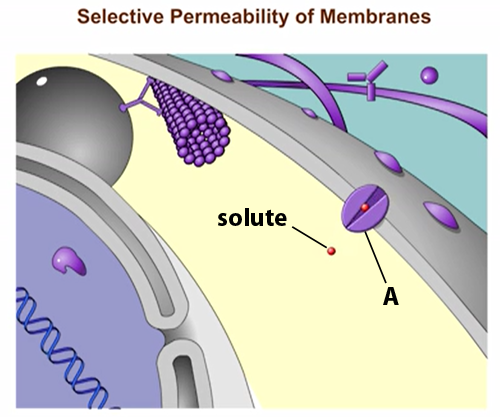
Structure A in the figure is a(n) _____.
receptor molecule
antibody
transport
protein
structural protein
enzyme
Transport Protein
The protein is allowing solute molecules to enter the cell
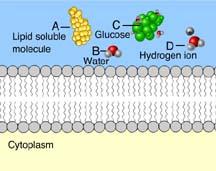
Which of these cannot rapidly pass directly through the phospholipids of the plasma membrane?
A only
C only
B only
D only
B, C, and D
B,C, and D
Ions, such as hydrogen ions, and hydrophilic molecules, such as water and glucose, cannot rapidly pass directly through the phospholipids of a plasma membrane. To move rapidly through the membrane, they must pass through membrane transport proteins.
Which of the following factors does not affect membrane permeability?
Temperature
The saturation of hydrocarbon tails in membrane
phospholipids
The polarity of membrane phospholipids
The
amount of cholesterol in the membrane
The polarity of membrane phospholipids
Phospholipids contain both a polar head and a nonpolar hydrocarbon tail, both of which are necessary for their ability to form membrane bilayers.
How can a lipid be distinguished from a sugar?
Lipids are mostly saturated.
Lipids are mostly
nonpolar.
A lipid dissolves in water.
A lipid is made up of
only hydrocarbons.
Lipids are mostly nonpolar
Lipids are nonpolar molecules, whereas sugars are polar.
True or false? Osmosis is a type of diffusion.
True
False
True
Osmosis is the diffusion of water across a selectively permeable membrane.
What property of dishwashing liquid (detergent) makes it useful to wash grease from pans?
Permeability
Amphipathic nature
Solubility in
water
Hydrophobic nature
Amphipathic Nature
Detergents form micelles around the grease, which are then washed away because the polar head groups facing outward on the micelle are water-soluble.
Which of the following particles could diffuse easily through a cell membrane?
Hydrogen ion (H+)
Sodium ion (Na+)
Oxygen (O2)
Glucose
Oxygen (O2)
Small nonpolar molecules such as oxygen can diffuse across cell membranes.
True or false? The water-soluble portion of a phospholipid is the
polar head, which generally consists of a glycerol molecule linked to
a phosphate group.
Hints
True
False
True
The hydrophilic, or water-loving, portion of a phospholipid is the polar head, whereas the hydrophobic portion is the nonpolar tail.
If a red blood cell is placed in a salt solution and bursts, what is
the tonicity of the solution relative to the interior of the cell?
Hints
Hypotonic
Osmotic
Hypertonic
Isotonic
Hypotonic
The salt concentration in the solution is lower than it is in the cell, so water enters the cell, causing it to burst.
What name is given to the process by which water crosses a selectively permeable membrane?
pinocytosis
phagocytosis
osmosis
passive transport
diffusion
Osmosis
Osmosis is the passive transport of water.
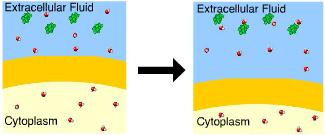
This cell is in a(n) _____ solution.
hypertonic
isotonic
hypotonic
hypertonic or
isotonic
hypotonic and isotonic
Hypertonic
There is a greater concentration of solute outside the cell.
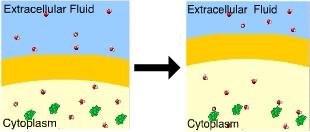
You know that this cell is in a(n) _____ solution because the cell _____.
isotonic ... neither lost nor gained water
hypertonic ... lost
water
hypertonic ... gained water
hypotonic ...
shrunk
hypotonic ... swelled
Hypotonic.......swelled
A cell will gain water when placed in a hypotonic solution.
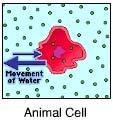
You know that this cell is in a(n) _____ solution because it _____.
hypertonic solution ... lost water
hypotonic ... is
turgid
hypertonic ... gained water
hypotonic ...
lysed
hypertonic ... lysed
Hypertonic solution..... lost water
A cell will lose water when placed in a hypertonic solution
A human cell placed into a hypertonic solution is likely to
burst as a result of osmosis.
lose water by
osmosis.
increase in size.
remain unchanged.
Lose water by osmosis
When a person is dehydrated, his or her IV fluids
-are not necessary, since a dehydrated person would not require IV
fluids.
-should be hypotonic, because if dehydrated, he or she
needs as much water as possible.
-should be isotonic, because
either a hypertonic or hypotonic IV would damage red blood
cells.
-should be hypertonic, because if dehydrated, he or she
probably needs salt as well.
should be isotonic, because either a hypertonic or hypotonic IV would damage red blood cells.
If you are going to bake potatoes, and your potatoes are soft and dehydrated, they can be soaked in __________ to make them more firm before baking.
a hypertonic solution such as distilled water
a hypotonic
solution such as tap water
a hypertonic solution made with
distilled water and a tablespoon of salt
an isotonic solution
A hypotonic solution such as tap water
A human cell placed in a hypotonic environment would
lose water through osmosis.
have no change in net water
balance.
shrivel up.
take up water through osmosis.
take up water through osmosis
A cell that neither gains nor loses water while sitting in a solution is probably sitting in
a hypotonic environment.
distilled water.
a hypertonic
environment.
an isotonic environment.
an isotonic environment
Paramecium is a genus of protists that lives in water. It has organelles called contractile vacuoles that continually eliminate the excess water gained through osmosis. Knowing that Paramecia gain water through osmosis, we can deduce that they normally live in
ice and very cold environments.
freshwater lakes and
ponds.
the ocean.
very salty environments.
freshwater lakes and ponds
Many bacteria and fungi have a difficult time surviving on our food if the food is very salty. The best explanation for this is
-that the salt in the food creates a hypertonic environment for the
bacteria and fungi.
-that bacteria and fungi cannot survive in a
hypotonic environment.
-that bacteria and fungi cannot survive in
an isotonic environment.
-that the salt in the food creates a
hypotonic environment for the bacteria and fungi.
that the salt in the food creates a hypertonic environment for the bacteria and fungi
If a single layer of phospholipids coats the water in a beaker (NOT A
BILAYER), which parts of the molecules will face up into the air?
-the hydrocarbon tails
-the phosphate heads
-both
the head and tails because the molecule is amphipathic
-the
glycolipid regions
-Hydrocarbon tails
The movement of tagged proteins in hybrid cells formed form the
fusion of a mice and human plasma membranes best illustrates
1.support for the fluid-mosaic model
2. flip flopping of
phospholipids
3.transport of small non-polar molecules into the
cell
4. the origins of Stuart Little
Support for the fluid-mosaic model
Glycoproteins and glycolipids are important for
cell-cell recognition
active transport
facilitated diffusion
energy reserves for the membrane
Cell-Cell recognition
Which of the following is an organelle found in a prokaryotic cell?
none, prokaryotes do not have organelles.
ribosome
DNA
plasma membrane
None, prokaryotes do not have organells
Autotrophs use which of the following to power cellular work
ATP
sunlight
glucose
CO2 and H2O
ATP
Which of the following is NOT a basic component of cell theory?
Viruses are the smallest type of cell.
All cells arise
from other cells
Cells are the most basic unit of life.
All living organisms are composed of cells.
Viruses are the smallest type of cell
Which of the following can be found in a bacterial chromosome?
DNA
histone proteins
chromatin
nucleoid region
DNA
The fluidity of membranes in a plant in cold weather may be
maintained by increasing the
-number of phospholipids with unsaturated hydrocarbon
tails.
-number of phospholipids with saturated hydrocarbon
tails.
-number of transmembrane proteins
-surface area to
volume ratio of the cell
number of phospholipids with unsaturated hydrocarbon tails
Facilitated diffusion of ions across a cellular membrane requires
_________; and the ions move ___________.
-channel proteins; down their electrochemical
gradient
-protein pumps; against the electrochemical
gradient
-channel proteins; down their concentration
gradient
-osmotic potentials; into the cell
Channel proteins; down their electrochemical gradient
Which of the following is NOT TRUE about osmosis?
-It is a passive process in cells without cell walls, but transport
across the cell wall requires energy.
-water moves into the cell
from a hypotonic solution
-it can occur rapidly though channel
proteins call aquaporins
-there is no net osmosis when cells are
in an isotonic solution.
It is a passive process in cells without cell walls, but transport across the cell wall requires energy
Why does the oxidation of organic compounds by molecular oxygen to produce CO2 and water release free energy?
-The covalent bonds in organic molecules and molecular oxygen have
more kinetic energy than the covalent bonds in water and carbon
dioxide.
-The oxidation of organic compounds can be used to make
ATP.
-Electrons are being moved from atoms that have a lower
affinity for electrons (such as C) to atoms with a higher affinity for
electrons (such as O).
-The covalent bond in O2 is unstable and
easily broken by electrons from organic molecules.
-The electrons
have a higher potential energy when associated with water and CO2 than
they do in organic compounds.
-Electrons are being moved from atoms that have a lower affinity for electrons (such as C) to atoms with a higher affinity for electrons (such as O).
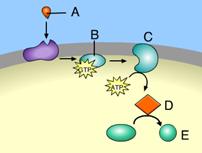
The ATP made during glycolysis is generated by
-the action of a kinase
enzyme.
-chemiosmosis.
-oxidative
phosphorylation.
-oxidation of NADH to NAD+.
-photophosphorylation.
The action of a kinase enzyme
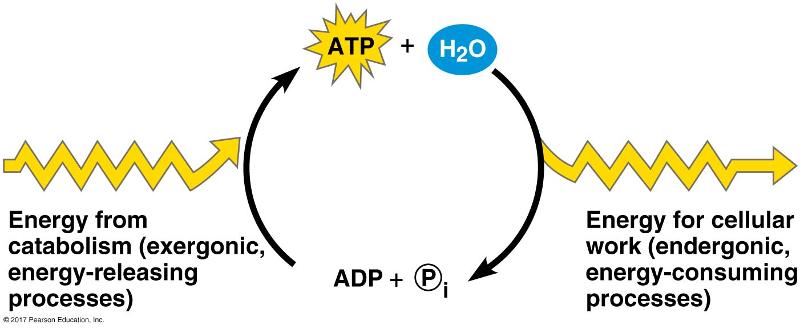
Which of the following is the most correct interpretation of the figure?
-ATP is a molecule that acts as an intermediary to store energy for
cellular work.
- i acts as a shuttle molecule to move energy from
ATP to ADP.
-Inorganic phosphate is created from organic
phosphate.
-Energy from catabolism can be used directly for
performing cellular work.
-ADP + i are a set of molecules that
store energy for catabolism.
-ATP is a molecule that acts as an intermediary to store energy for cellular work
The primary role of oxygen in cellular respiration is to
combine with carbon, forming CO2.
-act as an acceptor for electrons and hydrogen, forming
water.
-combine with lactate, forming pyruvate.
-catalyze
the reactions of glycolysis.
-yield energy in the form of ATP as
it is passed down the respiratory chain.
Act as an acceptor for electrons and hydrogen, forming water
A number of systems for pumping ions across membranes are powered by ATP. Such ATP-powered pumps are often called ATPases although they don't often hydrolyze ATP unless they are simultaneously transporting ions. Because small increases in calcium ions in the cytosol can trigger a number of different intracellular reactions, cells keep the cytosolic calcium concentration quite low under normal conditions, using ATP-powered calcium pumps. For example, muscle cells transport calcium from the cytosol into the membranous system called the sarcoplasmic reticulum (SR). If a resting muscle cell's cytosol has a free calcium ion concentration of 10-7 while the concentration in the SR is 10-2, then how is the ATPase acting?
-ATPase activity must be powering an inflow of calcium from the
outside of the cell into the SR.
-ATPase activity must be opening
a channel for the calcium ions to diffuse back into the SR along the
concentration gradient.
-ATPase activity must be pumping calcium
from the cytosol to the SR against the concentration
gradient.
-ATPase activity must be transferring i to the SR to
enable this to occur.
-ATPase activity must be routing calcium
ions from the SR to the cytosol, and then to the cell's environment.
ATPase activity must be pumping calcium from the cytosol to the SR against the concentration gradient.
The electrons carried by NADH and FADH2 can be
-transported into the matrix of the mitochondria.
-moved
between proteins in the inner membrane of the
mitochondria.
-pumped into the intermembrane space.
-transferred to the ATP synthase.
moved between proteins in the inner membrane of the mitochondria
What is the importance of fermentation to cellular metabolism?
It reduces NADH to NAD+ in the absence of O2.
It produces
ethanol or lactate for cellular respiration.
It uses pyruvate
to produce ethanol or lactate.
It oxidizes NADH to NAD+ in the
absence of O2.
It oxidizes NADH to NAD+ in the absence of O2.
Brown fat is special type of fat cell found in human babies and hibernating animals which helps these organisms maintain a high body temperature in hostile environments. Brown fat cells contain mitochondria that express an uncoupling protein located in the inner mitochondrial membrane, thermogenin, that serves as a passive transporter for protons. Brown fat cells can generate ATP and also generate a substantial amount of body heat. What mechanism best explains the role of uncoupling proteins in generating body heat?
-Oxidative phosphorylation produces excess heat due to the passive
transport of protons.
-The potential energy of the proton
gradient is converted to heat as the protons move down the
concentration gradient into the matrix.
-The potential energy of
the proton gradient is converted to heat as the protons move down the
concentration gradient into the intermembrane space.
-The energy
of the ATP synthase is converted to heat as the protons are pumped out
of the matrix by the uncoupling protein.
The potential energy of the proton gradient is converted to heat as the protons move down the concentration gradient into the matrix.
Animals inhale air containing oxygen and exhale air with less oxygen
and more carbon dioxide. After inhalation, the oxygen missing from the
air will mostly be found in
the carbon dioxide that is exhaled.
organic molecules.
lactate.
water.
ethanol.
Water
In the following redox reaction, _______ is oxidized and _______ is
reduced.
Glyceraldehyde 3-phosphate (G3P) + NAD+ + H+ + Pi →
1,3-Bisphosphoglycerate (BPG) + NADH
BPG; NADH + H+
NAD+; NADH + H+
G3P; NAD+
G3P; NADH
+ H+
G3P; NAD+
None of the above; the equation does not show a redox
reaction.
A molecule is oxidized when it loses electrons or
protons and is reduced when it gains electrons or protons. In this
reaction, G3P donates electrons and therefore is oxidized, while NAD+
accepts them and thus is reduced.