Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
EXAM 2 MATERIAL
front 1 Which of these enters the citric acid cycle? -G3P -glucose -acetyl CoA -NADH + H+ -pyruvate | back 1 Acetyl CoA |
front 2 In the citric acid cycle, ATP molecules are produced by _____. cellular respiration | back 2 -substrate-level phosphorylation |
front 3 Which of these is NOT a product of the citric acid cycle? ATP | back 3 -Acetyl CoA |
front 4 In the oxidation of pyruvate to acetyl CoA, one carbon atom is released as CO2. However, the oxidation of the remaining two carbon atoms—in acetate—to CO2 requires a complex, eight-step pathway—the citric acid cycle. Consider four possible explanations for why the last two carbons in acetate are converted to CO2 in a complex cyclic pathway rather than through a simple, linear reaction. Use your knowledge of the first three stages of cellular respiration to determine which explanation is correct. -More ATP is produced per CO2 released in cyclic processes than
in linear processes. | back 4 -It is easier to remove electrons and produce CO2 from compounds with three or more carbon atoms than from a two-carbon compound such as acetyl CoA Although it is possible to oxidize the two-carbon acetyl group of acetyl CoA to two molecules of CO2, it is much more difficult than adding the acetyl group to a four-carbon acid to form a six-carbon acid (citrate). Citrate can then be oxidized sequentially to release two molecules of CO2. |
front 5 Which molecule is metabolized in a cell to produce energy for performing work? Phosphate | back 5 -Glucose Glucose is used to produce high-energy ATP in a cell. |
front 6 True or false? The potential energy in an ATP molecule is derived mainly from its three phosphate groups. | back 6 True The three phosphate groups in an ATP molecule carry negative charges that strongly repel each other and give ATP a large amount of potential energy. |
front 7 Which process is not part of the cellular respiration pathway that produces large amounts of ATP in a cell? Glycolysis | back 7 Fermentation Fermentation is an alternate pathway used when oxygen levels are low. |
front 8 Which step of the cellular respiration pathway can take place in the absence of oxygen? Krebs cycle | back 8 Glycolysis Glycolysis can take place in the absence of oxygen; its product, pyruvate, enters the cellular respiration pathway or undergoes fermentation depending on the availability of oxygen. |
front 9 Into which molecule are all the carbon atoms in glucose ultimately incorporated during cellular respiration? NADH | back 9 Carbon Dioxide All of the carbon atoms in glucose are incorporated into carbon dioxide: Two molecules are formed as pyruvate is converted to acetyl CoA, and four molecules are formed during the Krebs cycle. |
front 10 Which of the following statements about the electron transport chain is true? The electron transport chain is the first step in cellular
respiration. | back 10 NADH and FADH2 donate their electrons to the chain The electrons lose energy as they move down the chain, and this energy is used to create a proton gradient that drives the synthesis of ATP. |
front 11 Which stage of glucose metabolism produces the most ATP? Krebs cycle | back 11 Electron transport and chemiosmosis Electron transport and chemiosmosis (oxidative phosphorylation) can yield around 26 molecules of ATP. |
front 12 True or false? The reactions that generate the largest amounts of ATP during cellular respiration take place in the mitochondria. True | back 12 TRUE Glycolysis takes place in the cytoplasm, whereas the Krebs cycle and the electron transport chain, which generate the largest amounts of ATP during cellular respiration, take place in the mitochondria. |
front 13 In mitochondrial electron transport, what is the direct role of O2? -to function as the final electron acceptor in the electron
transport chain | back 13 To function as the final electron acceptor in the electron transport chain The only place that O2 participates in cellular respiration is at the end of the electron transport chain, as the final electron acceptor. Oxygen's high affinity for electrons ensures its success in this role. Its contributions to driving electron transport, forming a proton gradient, and synthesizing ATP are all indirect effects of its role as the terminal electron acceptor. |
front 14 How would anaerobic conditions (when no O2 is present) affect the rate of electron transport and ATP production during oxidative phosphorylation? (Note that you should not consider the effect on ATP synthesis in glycolysis or the citric acid cycle.) Neither electron transport nor ATP synthesis would be
affected. | back 14 -Both electron transport and ATP synthesis would stop Oxygen plays an essential role in cellular respiration because it is the final electron acceptor for the entire process. Without O2, mitochondria are unable to oxidize the NADH and FADH2 produced in the first three steps of cellular respiration, and thus cannot make any ATP via oxidative phosphorylation. In addition, without O2 the mitochondria cannot oxidize the NADH and FADH2 back to NAD+ and FAD, which are needed as inputs to the first three stages of cellular respiration. |
front 15 NADH and FADH2 are both electron carriers that donate their electrons to the electron transport chain. The electrons ultimately reduce O2 to water in the final step of electron transport. However, the amount of ATP made by electrons from an NADH molecule is greater than the amount made by electrons from an FADH2 molecule. Which statement best explains why more ATP is made per molecule of NADH than per molecule of FADH2? -Fewer protons are pumped across the inner mitochondrial membrane
when FADH2 is the electron donor than when NADH is the electron
donor. | back 15 -Fewer protons are pumped across the inner mitochondrial membrane when FADH2 is the electron donor than when NADH is the electron donor. Electrons derived from the oxidation of FADH2 enter the electron transport chain at Complex II, farther down the chain than electrons from NADH (which enter at Complex I). This results in fewer H+ ions being pumped across the membrane for FADH2 compared to NADH, as this diagram shows. Thus, more ATP can be produced per NADH than FADH2. |
front 16 How many NADH are produced by glycolysis? 2 | back 16 2 Two NADH molecules are produced by glycolysis. |
front 17 In glycolysis, ATP molecules are produced by _____. photosynthesis | back 17 Substrate-level phosphorylation A phosphate group is transferred from glyceraldehyde phosphate to ADP. |
front 18 Which of these is NOT a product of glycolysis? NADH | back 18 FADH2 FADH2 is a product of the citric acid cycle. |
front 19 In glycolysis, what starts the process of glucose oxidation? NADPH | back 19 -ATP Some ATP energy is used to start the process of glucose oxidation. |
front 20 In glycolysis there is a net gain of _____ ATP. 2 | back 20 -2 It takes 2 ATP to produce 4 ATP. |
front 21 When a compound donates (loses) electrons, that compound becomes _________. Such a compound is often referred to as an electron donor. | back 21 Oxidized |
front 22 2. When a compound accepts (gains) electrons, that compound becomes __________. Such a compound is often referred to as an electron acceptor. | back 22 Reduced |
front 23 3. In glycolysis, the carbon-containing compound that functions as the electron donor is ______________. | back 23 -Glucose |
front 24 Once the electron donor in glycolysis gives up its electrons, it is oxidized to a compound called __________. | back 24 -Pyruvate |
front 25 5. ____________ is the compound that functions as the electron acceptor in glycolysis. | back 25 -NAD+ |
front 26 6. The reduced form of the electron acceptor in glycolysis is ___________. | back 26 -NADH |
front 27 Among the products of glycolysis, which compounds contain energy that can be used by other biological reactions? CO2 only | back 27 -pyruvate, ATP, NADH ATP is the main product of cellular respiration that contains energy that can be used by other cellular processes. Some ATP is made in glycolysis. In addition, the NADH and pyruvate produced in glycolysis are used in subsequent steps of cellular respiration to make even more ATP. |
front 28 Sort the statements into the appropriate bin depending on whether or not they correctly describe some aspect of substrate-level phosphorylation in glycolysis.
| back 28 CORRECT: -An enzyme is required in order for the reaction to occur -One of the substrates is a molecule derived from the breakdown of glucose -A bond must be broken between an organic molecule and a phosphate before ATP can form INCORRECT: -The enzymes involved in ATP synthesis must be attached to a membrane to produce ATP. -The phosphate group added to ADP to make ATP comes from free inorganic phosphate ions |
front 29 In muscle cells, fermentation produces _____. carbon dioxide, ethanol, NADH, and ATP | back 29 lactate and NAD+ These are the products of fermentation as it occurs in muscle cells. |
front 30 In fermentation _____ is reduced and _____ is oxidized. NADH ... lactate | back 30 -pyruvate......NADH The pyruvate from glycolysis is reduced to either lactate or ethanol, and NADH is oxidized to NAD+. |
front 31 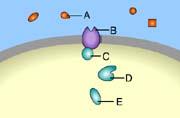 Part A C | back 31 B This is a receptor molecule. |
front 32 A signal transduction pathway is initiated when a _____ binds to a receptor. G protein | back 32 Signal Molecule The binding of a signal molecule to a receptor initiates a signal transduction pathway. |
front 33 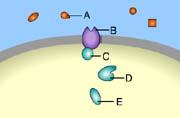 Which of these is a signal molecule? A | back 33 A This is a signal molecule |
front 34 A signal molecule is also known as a(n) _____. key | back 34 Ligand A ligand is a signal molecule. |
front 35 Which of these is the second of the three stages of cell signaling? gene activation | back 35 Transduction |
front 36 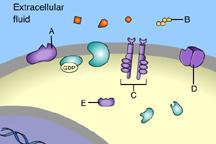 Which of these receptors is NOT a membrane receptor? A E D B C | back 36 E This receptor is not associated with the plasma membrane. |
front 37 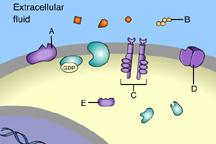 Which of these is a G-protein-linked receptor? E | back 37 A This is a G-protein-linked receptor. |
front 38 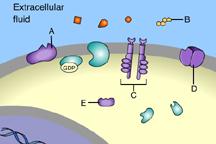 Which of these is a receptor tyrosine kinase? C | back 38 C This is a receptor tyrosine kinase. |
front 39 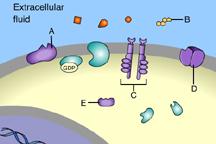 Which of these is an ion-channel receptor? D | back 39 D This receptor does form a channel. |
front 40 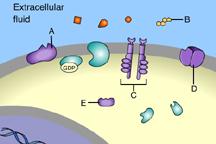 The binding of signal molecules to _____ results in the phosphorylation of tyrosines. A | back 40 C The binding of signal molecules to tyrosine-kinase receptors activates tyrosine-kinase enzymes, which phosphorylate tyrosines. |
front 41 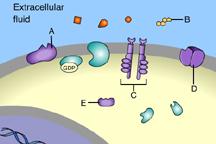 Which of these receptor molecules would allow Na+ to flow into the cell? A | back 41 D This is an ion-channel receptor. |
front 42 Which of these extracellular signal molecules could diffuse through a plasma membrane and bind to an intracellular receptor? glucose | back 42 Estrogen Nonpolar molecules can diffuse through the plasma membrane and bind to intracellular receptors |
front 43 A(n) _____ is an example of a signal molecule that can bind to an intracellular receptor and thereby cause a gene to be turned on or off. carbohydrate | back 43 Steroid Steroids bind to intracellular receptors, which can then bind to, and regulate, the expression of genes. |
front 44 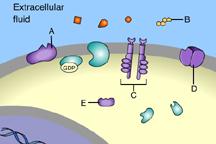 _____ is a signal molecule that binds to an intracellular receptor D | back 44 D Steroids are nonpolar and can diffuse through the plasma membrane |
front 45 Thyroid hormones bind to _____ receptors. steroid | back 45 Intracellular Thyroid hormones are able to pass through the plasma membrane. |
front 46 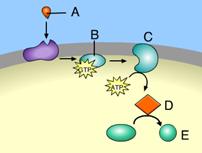 Which of these acts as a second messenger? D | back 46 D This is a second messenger. |
front 47 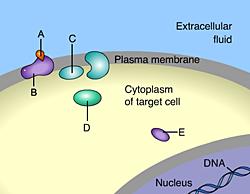 Which of these is responsible for initiating a signal transduction pathway?A B | back 47 A This is a signal molecule. The attachment of a signal molecule to a plasma membrane receptor initiates a signal transduction pathway. |
front 48 What role does a transcription factor play in a signal transduction pathway? -By binding to a plasma membrane receptor it initiates a
cascade. | back 48 By binding to DNA it triggers the Transcription of a specific gene This is the function of a transcription factor. |
front 49 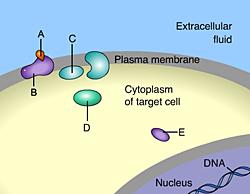 Which of these is a membrane receptor? A | back 49 B This is a receptor molecule. |
front 50 A signal transduction pathway is initiated when a _____ binds to a receptor. G protein | back 50 Signal Molecule The binding of a signal molecule to a receptor initiates a signal trane |
front 51 Which of these acts as a second messenger? protein kinase | back 51 Cyclic AMP Cyclic AMP can act as second messengers. |
front 52 Calcium ions that act as second messengers are stored in _____. endoplasmic reticula | back 52 Endoplasmic Reticula The ER stores calcium ions. |
front 53 _____ catalyzes the production of _____, which then opens an ion channel that releases _____ into the cell's cytoplasm. Adenylyl cyclase ... IP3 .... Ca2+ | back 53 Phospholipase C ... IP3 .... Ca2+ Phospholipase C cleaves IP3 from a membrane protein, and IP3 then binds to a calcium channel on the ER. |
front 54 A protein kinase activating many other protein kinases is an example of _____. deactivation | back 54 Amplification By activating many other molecules the initial signal is amplified. |
front 55 The cleavage of glycogen by glycogen phosphorylase releases _____. glucose-1-phosphate | back 55 Glucose-1 phosphate Glycogen is a polysaccharide composed of glucose monomers. |
front 56 Epinephrine acts as a signal molecule that attaches to _____ proteins. ion-channel receptor | back 56 G-protein-linked receptor Epinephrine acts via G-protein-linked receptors. |
front 57 Which of these is activated by calcium ions? G protein | back 57 Calmodulin Calmodulin is a calcium-binding protein. |
front 58 Which of these is NOT correct? Ion channels are found on both the plasma membrane and the
endoplasmic reticulum. | back 58 Cyclic AMP binds to calmodulin Calcium binds to calmodulin. |
front 59 A toxin that inhibits the production of GTP would interfere with the function of a signal transduction pathway that is initiated by the binding of a signal molecule to _____ receptors. intracellular | back 59 G-Protein Linked GTP activates G proteins. |
front 60 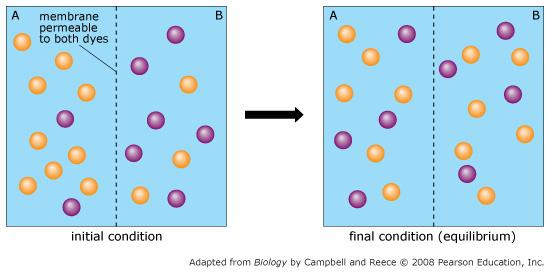 Orange dye moves independently of purple dye? | back 60 Always |
front 61 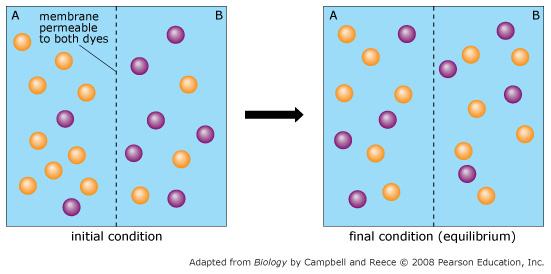 Concentration gradients exist that drive diffusion both dyes | back 61 Only before equilibrium is reached |
front 62 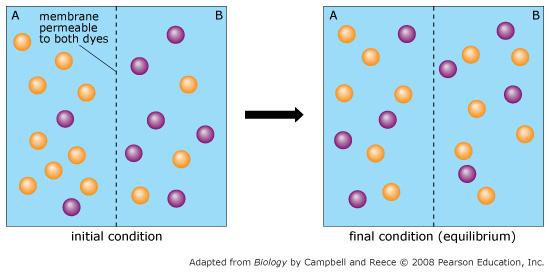 There is a net movement of orange dye from side A to B | back 62 Only before equilibrium is reached |
front 63 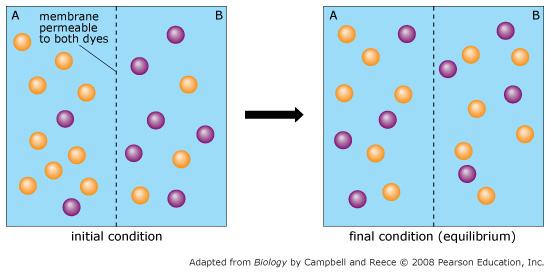 Purple dye moves only from side B to side A | back 63 Never |
front 64 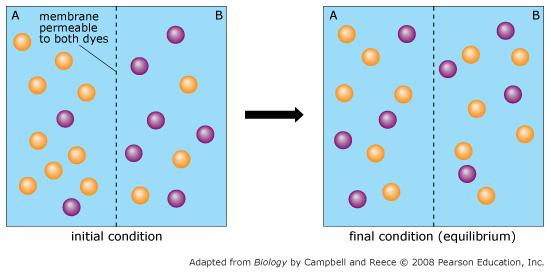 There is no movement of purple dye | back 64 Only at equilibrium |
front 65 Some solutes are able to pass directly through the lipid bilayer of a
plasma membrane, whereas other solutes require a transport protein or
other mechanism to cross between the inside and the outside of a cell.
The fact that the plasma membrane is permeable to some solutes but not
others is what is referred to as selective permeability. lipids | back 65 Lipids, Oxygen, Water, Carbon Dioxide
Some solutes pass readily through the lipid bilayer of a
cell membrane, whereas others pass through much more slowly, or
not at all. |
front 66 The majority of solutes that diffuse across the plasma membrane
cannot move directly through the lipid bilayer. The passive movement
of such solutes (down their concentration gradients without the input
of cellular energy) requires the presence of specific transport
proteins, either channels or carrier proteins. Diffusion through a
transport protein in the plasma membrane is called facilitated
diffusion.
| back 66 ONLY CHANNELS
ONLY CARRIERS
BOTH CHANNELS AND CARRIERS
|
front 67 Because ions carry a charge (positive or negative), their transport
across a membrane is governed not only by concentration gradients
across the membrane but also by differences in charge across the
membrane (also referred to as membrane potential). Together, the
concentration (chemical) gradient and the charge difference
(electrical gradient) across the plasma membrane make up the
electrochemical gradient. 1. The diffusion of Na+ ions into the cell is facilitated by the
Na+ concentration gradient across the plasma membrane. | back 67
The concentration gradient of K+ ions across the
membrane (higher K+ concentration inside) facilitates the
diffusion of K+ out of the cell. However, the electrical gradient
across the membrane (excess positive charge outside) impedes the
diffusion of K+ out of the cell. |
front 68 Which of the following statements is TRUE with regard to this animation? Potassium ions are transported down their concentration
gradient. | back 68 Both sodium and potassium ions are transported against their concentration gradients Both ions are transported from where their concentration is low to where their concentration is high, and the cell expends energy in the form of ATP to do it. |
front 69 Sort the phrases into the appropriate bins depending on whether they describe exocytosis, endocytosis, or both.
| back 69 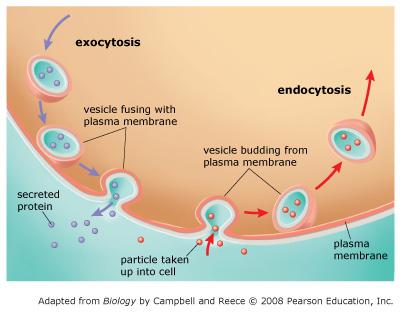 EXOCYTOSIS
ENDOCYTOSIS
BOTH
In exocytosis, substances are transported to the plasma membrane in vesicles derived from the endomembrane system. These vesicles fuse with the plasma membrane, releasing the enclosed substances outside the cell. In endocytosis, substances are taken into the cell by folding in of the plasma membrane and pinching off of the membrane to form a vesicle. Notice that in both exocytosis and endocytosis, the transported substances never actually cross the plasma membrane as they leave or enter the cell. |
front 70 Endocytosis moves materials _____ a cell via _____. into ... membranous vesicles | back 70 Into...... membranous vesicles The prefix "endo-" means "inward." |
front 71 You can recognize the process of pinocytosis when _____. the cell is engulfing a large particle | back 71 The cell is engulfing extracellular fluid Pinocytosis is "cell drinking." |
front 72 A white blood cell engulfing a bacterium is an example of _____. exocytosis | back 72 Phagocytosis Phagocytosis occurs when a cell engulfs a large particle. |
front 73 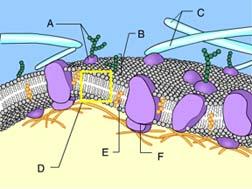 What is the function of Structure E? detection of environmental change | back 73 Stabilization of the phospholipids Cholesterol helps to stabilize the structure of the plasma membrane. |
front 74 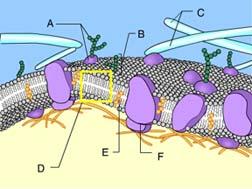 Identify Structure D. protein | back 74 Phospholipid bilayer of membrane Phospholipids can be recognized by the presence of a head and two tails. |
front 75 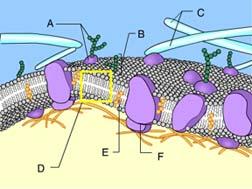 Identify Structure A. protein | back 75 Glycoprotein Structure A is composed of both a carbohydrate and a protein. |
front 76 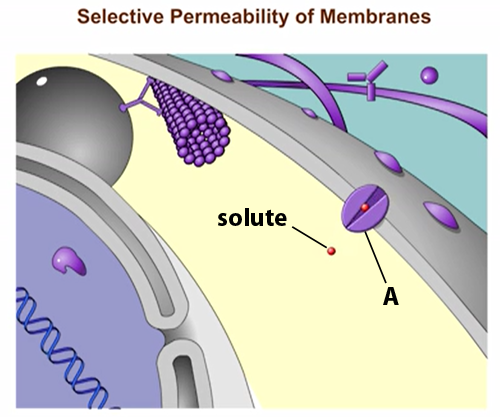 Structure A in the figure is a(n) _____. receptor molecule | back 76 Transport Protein The protein is allowing solute molecules to enter the cell |
front 77 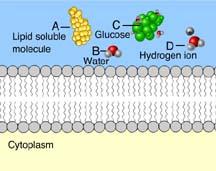 Which of these cannot rapidly pass directly through the phospholipids of the plasma membrane? A only | back 77 B,C, and D Ions, such as hydrogen ions, and hydrophilic molecules, such as water and glucose, cannot rapidly pass directly through the phospholipids of a plasma membrane. To move rapidly through the membrane, they must pass through membrane transport proteins. |
front 78 Which of the following factors does not affect membrane permeability? Temperature | back 78 The polarity of membrane phospholipids Phospholipids contain both a polar head and a nonpolar hydrocarbon tail, both of which are necessary for their ability to form membrane bilayers. |
front 79 How can a lipid be distinguished from a sugar? Lipids are mostly saturated. | back 79 Lipids are mostly nonpolar Lipids are nonpolar molecules, whereas sugars are polar. |
front 80 True or false? Osmosis is a type of diffusion. True | back 80 True Osmosis is the diffusion of water across a selectively permeable membrane. |
front 81 What property of dishwashing liquid (detergent) makes it useful to wash grease from pans? Permeability | back 81 Amphipathic Nature Detergents form micelles around the grease, which are then washed away because the polar head groups facing outward on the micelle are water-soluble. |
front 82 Which of the following particles could diffuse easily through a cell membrane? Hydrogen ion (H+) | back 82 Oxygen (O2) Small nonpolar molecules such as oxygen can diffuse across cell membranes. |
front 83 True or false? The water-soluble portion of a phospholipid is the
polar head, which generally consists of a glycerol molecule linked to
a phosphate group. True | back 83 True The hydrophilic, or water-loving, portion of a phospholipid is the polar head, whereas the hydrophobic portion is the nonpolar tail. |
front 84 If a red blood cell is placed in a salt solution and bursts, what is
the tonicity of the solution relative to the interior of the cell? Hypotonic | back 84 Hypotonic The salt concentration in the solution is lower than it is in the cell, so water enters the cell, causing it to burst. |
front 85 What name is given to the process by which water crosses a selectively permeable membrane? pinocytosis | back 85 Osmosis Osmosis is the passive transport of water. |
front 86 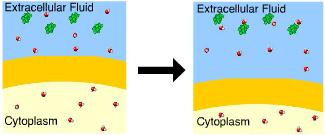 This cell is in a(n) _____ solution. hypertonic | back 86 Hypertonic There is a greater concentration of solute outside the cell. |
front 87 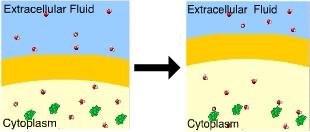 You know that this cell is in a(n) _____ solution because the cell _____. isotonic ... neither lost nor gained water | back 87 Hypotonic.......swelled A cell will gain water when placed in a hypotonic solution. |
front 88 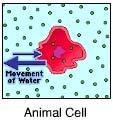 You know that this cell is in a(n) _____ solution because it _____. hypertonic solution ... lost water | back 88 Hypertonic solution..... lost water A cell will lose water when placed in a hypertonic solution |
front 89 A human cell placed into a hypertonic solution is likely to burst as a result of osmosis. | back 89 Lose water by osmosis |
front 90 When a person is dehydrated, his or her IV fluids -are not necessary, since a dehydrated person would not require IV
fluids. | back 90 should be isotonic, because either a hypertonic or hypotonic IV would damage red blood cells. |
front 91 If you are going to bake potatoes, and your potatoes are soft and dehydrated, they can be soaked in __________ to make them more firm before baking. a hypertonic solution such as distilled water | back 91 A hypotonic solution such as tap water |
front 92 A human cell placed in a hypotonic environment would lose water through osmosis. | back 92 take up water through osmosis |
front 93 A cell that neither gains nor loses water while sitting in a solution is probably sitting in a hypotonic environment. | back 93 an isotonic environment |
front 94 Paramecium is a genus of protists that lives in water. It has organelles called contractile vacuoles that continually eliminate the excess water gained through osmosis. Knowing that Paramecia gain water through osmosis, we can deduce that they normally live in ice and very cold environments. | back 94 freshwater lakes and ponds |
front 95 Many bacteria and fungi have a difficult time surviving on our food if the food is very salty. The best explanation for this is -that the salt in the food creates a hypertonic environment for the
bacteria and fungi. | back 95 that the salt in the food creates a hypertonic environment for the bacteria and fungi |
front 96 If a single layer of phospholipids coats the water in a beaker (NOT A
BILAYER), which parts of the molecules will face up into the air? | back 96 -Hydrocarbon tails |
front 97 The movement of tagged proteins in hybrid cells formed form the
fusion of a mice and human plasma membranes best illustrates | back 97 Support for the fluid-mosaic model |
front 98 Glycoproteins and glycolipids are important for | back 98 Cell-Cell recognition |
front 99 Which of the following is an organelle found in a prokaryotic cell? | back 99 None, prokaryotes do not have organells |
front 100 Autotrophs use which of the following to power cellular work | back 100 ATP |
front 101 Which of the following is NOT a basic component of cell theory? | back 101 Viruses are the smallest type of cell |
front 102 Which of the following can be found in a bacterial chromosome? | back 102 DNA |
front 103 The fluidity of membranes in a plant in cold weather may be
maintained by increasing the | back 103 number of phospholipids with unsaturated hydrocarbon tails |
front 104 Facilitated diffusion of ions across a cellular membrane requires
_________; and the ions move ___________. | back 104 Channel proteins; down their electrochemical gradient |
front 105 Which of the following is NOT TRUE about osmosis? -It is a passive process in cells without cell walls, but transport
across the cell wall requires energy. | back 105 It is a passive process in cells without cell walls, but transport across the cell wall requires energy |
front 106 Why does the oxidation of organic compounds by molecular oxygen to produce CO2 and water release free energy? -The covalent bonds in organic molecules and molecular oxygen have
more kinetic energy than the covalent bonds in water and carbon
dioxide. | back 106 -Electrons are being moved from atoms that have a lower affinity for electrons (such as C) to atoms with a higher affinity for electrons (such as O). |
front 107 The ATP made during glycolysis is generated by | back 107 The action of a kinase enzyme |
front 108 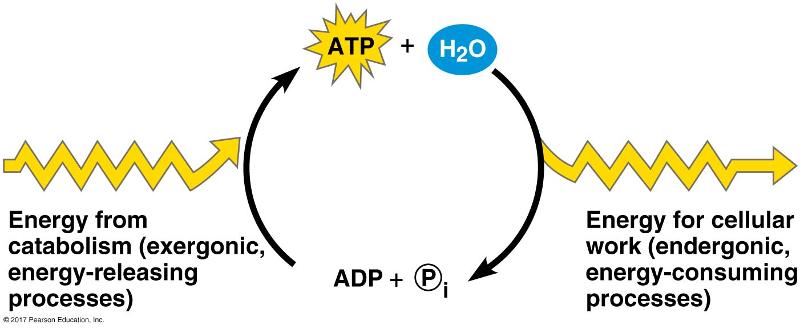 Which of the following is the most correct interpretation of the figure? -ATP is a molecule that acts as an intermediary to store energy for
cellular work. | back 108 -ATP is a molecule that acts as an intermediary to store energy for cellular work |
front 109 The primary role of oxygen in cellular respiration is to | back 109 Act as an acceptor for electrons and hydrogen, forming water |
front 110 A number of systems for pumping ions across membranes are powered by ATP. Such ATP-powered pumps are often called ATPases although they don't often hydrolyze ATP unless they are simultaneously transporting ions. Because small increases in calcium ions in the cytosol can trigger a number of different intracellular reactions, cells keep the cytosolic calcium concentration quite low under normal conditions, using ATP-powered calcium pumps. For example, muscle cells transport calcium from the cytosol into the membranous system called the sarcoplasmic reticulum (SR). If a resting muscle cell's cytosol has a free calcium ion concentration of 10-7 while the concentration in the SR is 10-2, then how is the ATPase acting? -ATPase activity must be powering an inflow of calcium from the
outside of the cell into the SR. | back 110 ATPase activity must be pumping calcium from the cytosol to the SR against the concentration gradient. |
front 111 The electrons carried by NADH and FADH2 can be -transported into the matrix of the mitochondria. | back 111 moved between proteins in the inner membrane of the mitochondria |
front 112 What is the importance of fermentation to cellular metabolism? It reduces NADH to NAD+ in the absence of O2. | back 112 It oxidizes NADH to NAD+ in the absence of O2. |
front 113 Brown fat is special type of fat cell found in human babies and hibernating animals which helps these organisms maintain a high body temperature in hostile environments. Brown fat cells contain mitochondria that express an uncoupling protein located in the inner mitochondrial membrane, thermogenin, that serves as a passive transporter for protons. Brown fat cells can generate ATP and also generate a substantial amount of body heat. What mechanism best explains the role of uncoupling proteins in generating body heat? -Oxidative phosphorylation produces excess heat due to the passive
transport of protons. | back 113 The potential energy of the proton gradient is converted to heat as the protons move down the concentration gradient into the matrix. |
front 114 Animals inhale air containing oxygen and exhale air with less oxygen
and more carbon dioxide. After inhalation, the oxygen missing from the
air will mostly be found in organic molecules. | back 114 Water |
front 115 In the following redox reaction, _______ is oxidized and _______ is
reduced. BPG; NADH + H+ | back 115 G3P; NAD+ None of the above; the equation does not show a redox
reaction. |