Differentiate:
Element
Compound
Mixture
Element - Single type of atom
Compound - Multiple elements on one
Mixture - Combination
Classify as element, compound, mixture: Soda, NO, Ar, Zn, NaCl
Element: Ar, Zn
Compound: NaCl, NO
Mixture: Soda
Classify as homogenous or heterogenous: Fruit salas, honey, bronze, smog, vodka
Homogenous: Vodka, honey, bronze
Heterogenous: Fruit salad, smog
A sample of seawater consists of water, sodium ions, chloride ions, and several other dissolved salts. These ions and salts are evenly distributed throughout the water.
Which term or terms could be used to describe this sample of seawater?
a. Mixture
b. Heterogeneous mixture
c. Homogeneous mixture
d. Solution
e. Pure chemical substance
f. Compound
g. Element
Mixture, homogeneous mixture, solution
A copper foil is made from copper, absolutely free of impurities.
Which term or terms could be used to describe this sample of copper?
a. Mixture
b. Heterogeneous mixture
c. Homogeneous mixture
d. Solution
e. Pure chemical substance
f. Compound
g. Element
Pure chemical substance, element
A sample of concrete contains cement, gravel, crushed rocks, sand, and water. Each of these contains different metals and minerals and hence has different colors and different properties.
Which term or terms could be used to describe this sample of concrete?
a. Mixture
b. Heterogeneous mixture
c. Homogeneous mixture
d. Solution
e. Pure chemical substance
f. Compound
g. Element
Mixture, heterogenous mixture
A glass jar contains pure sodium chloride.
Which term or terms could be used to describe the contents of this glass jar?
a. Mixture
b. Heterogeneous mixture
c. Homogeneous mixture
d. Solution
e. Pure chemical substance
f. Compound
g. Element
Pure chemical substance, compound
Intensive vs Extensive for Zinc:
Density of 7.13g/cm3
Melting point 419.58C
Mass of 63.74g
Volume of 8.94mL
Bluish gray in color
Intensive: Density of 7.13g/cm3, Melting point 419.58C, Bluish gray in color
Extensive: Mass of 63.74g, Volume of 8.94mL
You are given a sample resembling zinc. Which of the following properties could be used to help determine whether the sample is really zinc?
The density of the sample
The volume of the sample
The melting point of the sample
The density of the sample
The melting point of the sample
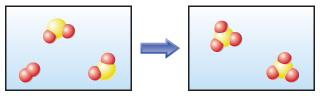
Chemical or physical change?
Chemical change
The molecules after the change are different than the molecules before the change.
A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen.
Based on these observations, can we determine whether solid A is element or compound?
a. Element
b. Compound
c. Can not determine
b. Compound
A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen.
Based on these observations, can we determine whether solid B is element or compound?
a. Element
b. Compound
c. Can not determine
c. Can not determine
A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen.
Based on these observations, can we determine whether gas C is element or compound?
a. Element
b. Compound
c. Can not determine
b. Compound
A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen.
Explain your conclusions for each substance.
When solid carbon is burned in excess oxygen gas, the two elements combine to form a gaseous compound, carbon dioxide. Clearly substance C is this compound. Since C is produced when A is heated in the absence of oxygen (from air), both the carbon and oxygen in C must have been present in A originally. A is, therefore, a compound composed of two or more elements chemically combined. Without more information on the chemical or physical properties of B, we cannot determine absolutely whether it is an element or a compound. However, few if any elements exist as white solids, so B is probably also a compound.
7-9
...