1) Which of the following is reflective of the phrase "the whole is greater than the sum of its parts"?
A) high-throughput technology
B) natural selection
C)
emergent properties
D) reductionism
E) feedback regulations
C
2) Sometimes atoms form molecules by sharing two pairs of valence electrons. When this occurs, the atoms are said to be joined by 2) _______
A) a hydrogen bond.
B) a protonic bond.
C) an
electronegative bond.
D) a double covalent bond.
E) a complex bond.
D
3) Which of these is a correct representation of the hierarchy of biological organization from least to most complex? 3) _______
A) molecule, small intestine, large intestine, intestinal tissue,
digestive system, organism
B) molecule, digestive system,
digestive cell organelle, small intestine, large intestine, intestinal
cell, organism C) molecule, intestinal cell organelle, intestinal
cell, intestinal tissue, digestive system, organism
D) organelle
of a stomach cell, digestive system, large intestine, small intestine,
intestinal tissue, organism
E) organelle of an intestinal cell,
digestive system, small intestine, large intestine, intestinal tissue, organism
C
4) Evolution is biology's core theme that ties together all the other themes. This is because evolution explains 4) _______
A) why distantly related organisms sometimes resemble each
other.
B) how organisms become adapted to their environment
through the differential reproductive success of varying
individuals.
C) explains why some organisms have traits in
common.
D) the unity and diversity of life.
E) all of the above
E
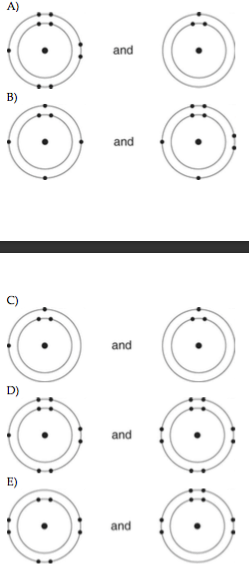
5) Which of the following pairs of atoms would be most likely to form an ionic bond?
A
B
C
D
E
A
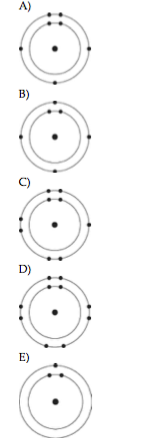
6) Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A
B
C
D
E
E
7) Prokaryotes are classified as belonging to two different domains.
What are the domains? A) Bacteria and Protista
B) Archaea and Monera
C) Eukarya and Monera
D) Bacteria and Eukarya
E) Bacteria and Archaea
E
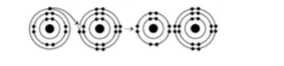
8) What is the atomic number of the cation formed in the reaction illustrated in Figure 2.3?
A) 11
B) 16
C) 10
D) 8 E) 1
A
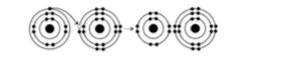
9) What results from the chemical reaction illustrated in Figure 2.3?
A) a cation with a net charge of +1
B) a cation with a net
charge of -1
C) an anion with a net charge of +1
D) an anion with a net charge of -1 E) A and D
E
10) When blood glucose level rises, the pancreas secretes insulin, and as a result blood glucose level declines. When blood glucose level is low, the pancreas secretes glucagon, and as a result blood glucose level rises. Such regulation of blood glucose level is the result of
A) catalytic feedback.
B) protein-protein interactions. C) bioinformatic regulation.
D) positive feedback.
E) negative feedback.
E
11) The lowest level of biological organization that can perform all
the activities required for life is the A) tissue–for example, nervous
tissue.
B) organ system–for example, the reproductive system.
C) organelle–for example, a chloroplast.
D) organism–for example, an amoeba, dog, human, or maple tree.
E) cell–for example, a skin cell.
E
12) Which of the following results from a transfer of electron(s) between atoms?
A) nonpolar covalent bond
B) ionic bond
C) hydrophobic interaction
D) hydrogen bond
E) polar covalent bond
B
13) Why is it important that an experiment include a control
group?
A) A control group is required for the development of an
"if, then" statement.
B) Without a control group,
there is no basis for knowing if a particular result is due to the
variable being tested or to some other factor.
C) A control
group assures that an experiment will be repeatable.
D) The
control group is the group that the researcher is in control of; it is
the group in which the researcher predetermines the nature of the results.
E) The control group provides a reserve of experimental subjects.
B
14) Which of the following sequences represents the hierarchy of biological organization from the least to the most complexlevel? 14)______
A) organism, community, biosphere, molecule, tissue, organ
B)
molecule, cell, organ system, population, ecosystem, biosphere
C) organelle, tissue, biosphere, ecosystem, population, organism
D) ecosystem, cell, population, tissue, organism, organ system
E) cell, community, population, organ system, molecule, organelle
B
15) Which of the following properties or processes do we associate
with living things? A) responding to the environment
B) growth
and reproduction
C) energy processing
D) evolutionary adaptations
E) all of the above
E
16) Which of the following is not considered to be a weak molecular
interaction? A) a covalent bond
B) a hydrogen bond
C) a
van der Waals interaction
D) an ionic bond in the presence of water
E) A and B only
A
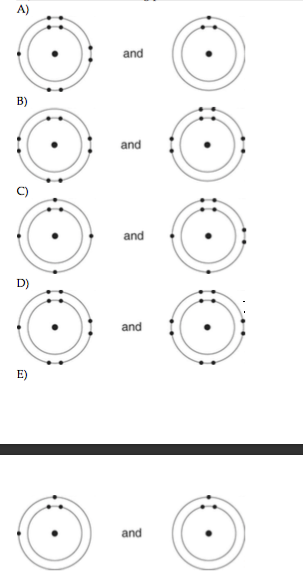
17) Which of the following pairs of atoms would be most likely to form a covalent bond?
A
B
C
D
E
C
18) A water sample from a hot thermal vent contained a single-celled organism that had a cell wall but lacked a nucleus. What is its most likely classification?
A) Protista
B) Eukarya
C) Animalia
D) Fungi
E) Archaea
E
19) Once labor begins in childbirth, contractions increase in intensity and frequency until delivery. The increasing labor contractions of childbirth are an example of
A) enzymatic catalysis.
B) a bioinformatic system.
C) feedback inhibition.
D) negative feedback.
E)
positive feedback.
E
20) A group of molecular biologists is trying to synthesize a new artificial compound to mimic the effects of a known hormone that influences sexual behavior. They have turned to you for advice. Which of the following compounds is most likely to mimic the effects of the hormone? 20) ______
A) a compound with the same three-dimensional shape as part of the
hormone
B) a compound with the same number of carbon atoms as
the hormone
C) a compound with the same molecular mass (measured
in daltons) as the hormone D) a compound with the same number of
orbital electrons as the hormone
E) a compound with the same
number of hydrogen and nitrogen atoms as the hormone
A
21) For most ecosystems __________ is (are) the ultimate source of energy, and energy leaves the ecosystem in the form of __________.
A) heat; light
B) sunlight; heat
C) producers; consumers
D) plants; animals
E) plants; heat
B
22) In terms of the hierarchical organization of life, a bacterium is at the __________ level of organization, whereas a
human is at the __________ level of organization.
A) organelle; organ system
B) tissue; organism
C) single
organelle; organism
D) single-celled organism; multicellular organism
E) single tissue; multicellular organism
D
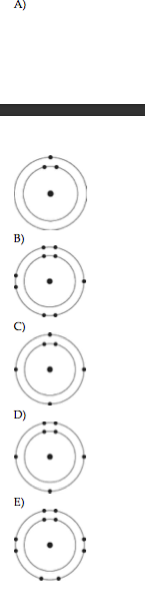
23) Which one of the atoms shown would be most likely to form an anion with a charge of -1?
a
b
c
d
e
B
24) A localized group of organisms that belong to the same species is called a
A) family.
B) population.
C) biosystem.
D) community.
E) ecosystem.
B
25) What is a hypothesis?
A) a fact based on quantitative data
that is falsifiable
B) a fact based on qualitative data that is
testable
C) a verifiable observation sensed directly, or sensed
indirectly with the aid of scientific instrumentation
D) the same thing as an unproven theory
E) a tentative
explanation that can be tested and is falsifiable
E
26) Which of the following molecules contains the strongest polar covalent bond?
A)H2O
B)H2
C)O2
D)CH4
E)CO2
A
27) Which of the following is (are) true of natural selection?
A) results in descent with modification
B) requires genetic
variation
C) involves differential reproductive success
D) B and C only
E) A, B, and C
E
28) Which branch of biology is concerned with the naming and classifying of organisms?
A) informatics
B) genomics
C) taxonomy
D) evolution
E) schematic biology
C
29) What is the difference between covalent bonds and ionic
bonds?
A) Covalent bonds involve the sharing of protons between
atoms, and ionic bonds involve the sharing of electrons between
atoms.
B) Covalent bonds involve the sharing of protons between
atoms, and ionic bonds involve the sharing of neutrons between
atoms.
C) Covalent bonds involve the sharing of neutrons between
atoms, and ionic bonds involve the sharing of electrons between
atoms.
D) Covalent bonds involve the sharing of electrons
between atoms, and ionic bonds involve the electrical attraction
between atoms.
E) Covalent bonds involve the transfer of
electrons between atoms, and ionic bonds involve the sharing of
neutrons between atoms.
D
30) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living matter?
A) oxygen, hydrogen, calcium, sodium
B) carbon, sulfur, phosphorus, hydrogen
C) carbon, sodium, chlorine, nitrogen
D) carbon, hydrogen,
nitrogen, oxygen
E) carbon, oxygen, sulfur, calcium
D
31) Charles Darwin proposed a mechanism for descent with modification which stated that organisms of a particular species are adapted to their environment when they possess
A) inheritable traits that decrease their survival and reproductive
success in the local environment.
B) non-inheritable traits that
enhance their survival and reproductive success in the local
environment. C) non-inheritable traits that enhance their reproductive
success in the local environment.
D) non-inheritable traits that
enhance their survival in the local environment.
E) inheritable
traits that enhance their survival and reproductive success in the
local environment.
E
32) Which of the following explains most specifically the attraction of water molecules to one another?
A) ionic bond
B) hydrophobic interaction
C) polar
covalent bond
D) nonpolar covalent bond
E) hydrogen bond
E