What is the electrophile in a Friedel-Crafts reaction?
carbocation
How is the alkyl electrophile formed during Friedel-Crafts alkylation?
Lewis acid catalyst is used to remove the halogen from an alkyl halide, creating a cation which is then attacked by the pi electrons in the aromatic system.
How is the aromatic ring reformed during the electrophilic aromatic substitution using Friedel-Crafts alkylation and acylation?
water or the conjugate base of the acid catalyst attacks the hydrogen on the same carbon as the substituent being added, causing the electrons to fall back into the ring
- Rearrangement of substituents
- Polysubstitution
- Cannot use vinyl halides
- Cannot be performed on deactivated ring
These are all drawbacks of:
Friedel-Crafts alkylation
CN, HOSO2, NO2 act as what kind of substituents on a benzene ring? What effect does this have on substituents being added to the ring? Are halogens considered part of this group?
deactivating, withdraws electron density from ortho positions causing the substituents to direct to the meta position preferentially, halogens are considered deactivating bc they are slower than benzene but you can perform Friedel-Crafts on a halobenzene and it will direct ortho-para.
What can cause a substituent to prefer the para position over the ortho position?
steric hindrance
In the nitration of methyl benzoate, how is the nitronium ion intermediate formed?
nitric acid is protonated by the sulfuric acid catalyst, which causes the formation of a small, stable leaving group: water
What effect does temperature have on nitration products?
an increase in temperature correlates to an increased number of additions to a benzene ring. To obtain a monosubstituted product, the reaction must take place at cold temperatures.
What is the relationship between borneol and isoborneol?
diastereomers
How is camphor able to form two stereoisomers?
planar geometry of the C=O double bond allows it to be attacked from above and below
A bottom-side attack of camphor using sodium borohydride will cause the ___________ isomer to form.
Isoborneol
A top-face attack of camphor using sodium borohydride will cause the ___________ isomer to form.
Borneol
Isoborneol is converted to camphor by _____________ using sodium hypochlorite.
oxidation
What mechanism is used to oxidize isoborneol to camphor?
E2 elimination
How many chiral centers do isoborneol and borneol possess respectively?
3
What is the chiral conformation of borneol?
R,S
What is the chiral conformation of isoborneol?
R,R
In order to perform the oxidation of camphor using household bleach, what was done to sodium hypochlorite (NaOCl)?
it was converted to the oxidizing agent hypochlorous acid using glacial acetic acid
What is considered the nucleophile in a Grignard reagent
the carbon attached to the magnesium
Nucleophiles are Lewis _________.
bases
What does the carbon nucleophile in a Grignard reagent attack?
the partially positive carbon in the C=O bond
Why is it so important to perform a Grignard Reaction using anhydrous conditions?
carbon nucleophiles are aggressive and will react with atmospheric moisture to form MgBrOH
The purpose of HCl in Grignard reactions is to
protonate the oxygen on the product to form an alcohol
What test is performed to detect the presence of an aldehyde?
Tollen's test
What test is performed to detect the presence of a methyl ketone?
Iodoform test
What aldehyde will produce a positive Iodoform?
ethanal, also known as acetaldehyde
The iodoform reaction proceeds via
alpha elimination
What reaction uses: Ag(NH3)2 under basic conditions?
Tollen's test
In Iodoform reactions, what does iodine substitute?
all 3 alpha hydrogens on the methyl group
What does a positive Iodoform test produce?
bright yellow precipitate
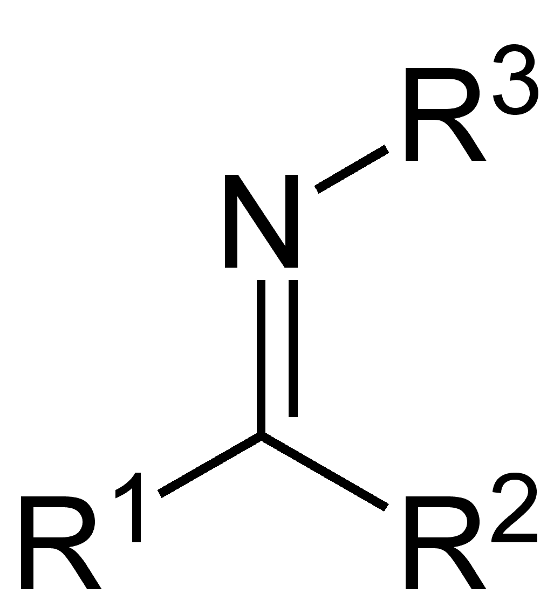
What is this compound called?
imine
What is the function of the acid catalyst in imine formation?
the elimination of water to form the double bond between carbon and nitrogen.
What happens if you use NHO3 and H2SO4 on an aniline ring?
the lone pair on the NH2 group is a Lewis base and will react with the Lewis acid catalyst
True or False: it is ok to add boiling chips to a round bottom flask that is already being heated
False
True or False: If a chemical is splashed on your face, remove your goggles before washing
False
What is the purpose of reflux?
allows the reaction to be heated at the boiling point of the reactants which increases the overall rate of the ration without loss of product from evaporation, potentially increasing the overall yield
What is the purpose of distillation?
uses a condenser to collect and isolate a pure product from a mixture of reactants based on differences in boiling points
What is the purpose of reacting an aldehyde/ketone with semicarbazone or 2,4-DNP?
convert liquid aldehydes and ketones to a solid for the purposes of melting point comparison
Semicarbazide possesses two -NH2 groups, one that is alpha to the carbonyl carbon (C=O) and one that is beta. Why is the alpha group less reactive?
electrons are tied up in resonance with the carbonyl group
Why is the carbanion formed during aldol condensation stable?
resonance structure called an enolate
What reagent is used to form the enolate during aldol condensation and what does it attack?
NaOH, alpha hydrogens
Why do aldol products that form from aromatic aldehydes/ketones spontaneously dehydrate?
because they are conjugated and extremely stable
How do you avoid creating the Cannizarro side product that is sometimes formed during an aldol condensation?
combine the reactants together before adding base so that benzaldehyde does not react with it
What does the excess benzaldehyde react with during a Cannizarro side product formation of an aldol condensation, and what does it form?
NaOH, benzoic acid and benzyl alcohol
What is Fisher Esterification?
the acid-catalyzed and reversible reaction between a carboxylic acid and an alcohol to create an ester
According to LeChatelier's Principle, how would you tip the reversible Fisher Esterification reaction toward the formation of product?
removal of product as it is formed, heating the solution, and increasing the concentration of the reactants
What is the role of acetic acid and sulfuric acid in Fischer Esterification?
acetic acid is the carboxylic acid and sulfuric acid is the catalyst. When combined they form a carbocation that will react with alcohol. Sulfuric acid also serves to remove the water byproduct, shifting the equilibrium in favor of the products
What is the purpose of washing the Fisher Esterification product in sodium bicarbonate?
its a weak base which will quench any excess acid to its conjugate base (a salt) which will precipitate out as a solid and can be easily removed
What can you calculate using this equation?
[(2xC+2)+N-H-X] / 2
degrees of unsaturation
What kind of spectroscopy can be used to determine functional groups?
IR spectroscopy
The number of signals (peaks) in Proton-NMR is equivalent to the number of individual or sets of
unique hydrogens
The __________ reflects the number of H's associated with a particular peak
integration
Ppm, or parts per million, corresponds with bond polarity. It is also called
chemical shift
What will have a Carbon NMR peak in the 200 ppm range?
C=O
What is generally considered the aliphatic range for Carbon NMR?
20-140 ppm
What is generally considered the aromatic range for Carbon NMR?
110-170 ppm
What is generally considered the C-X or C-O range for Carbon NMR?
45-90 ppm
What is generally considered the COOH range for Proton NMR?
9.8-13 ppm
What is generally considered the aldehyde range for Proton NMR?
9-10 ppm
What is generally considered the aromatic range for Proton NMR?
6.5-8.5 ppm
What is generally considered the amine range for Proton NMR?
0.7-2.7 ppm
What is generally considered the ~OH range for Proton NMR?
1-5 ppm
What is generally considered the C-O,C-X,C-NR2 range for Proton NMR?
2.5-4.3 ppm
What is generally considered the amide range for Proton NMR?
5-8 ppm
What is generally considered the alkene range for Proton NMR?
4.4-7 ppm
What is generally considered the ketone range for Proton NMR?
2-3 ppm
What groups will have the highest Proton NMR chemical shifts?
carboxylic acids and aldehydes
What 3 clues can Proton NMR give about the structure of an unknown?
chemical shift provides functional group clues, N+1 (splitting pattern) can give connectivity clues, and integration can determine the number of unique hydrogens
How many degrees of unsaturation does an aromatic ring have?
4
What type of functional group will have an IR peak at 1700?
C=O
Where will aromatic ring peaks show up on IR?
1500-1600
Where will N-H (amine) groups show up on IR?
3300-3500
An aldehyde will have twin peaks at ______ and ______ and a long, stretched out peak at ______.
2700, 2800, 1700
C-H bonds in aromatic rings will have IR peaks around
3000-3100
Where will nitriles (C triple bonded to N) show up on IR?
2200
What is considered the aliphatic range of Proton NMR?
1-4.5 ppm
What is considered the aromatic range of Proton NMR?
6.5-8.5 ppm
A Wittig reaction uses a strong base (such as NaOH) to pull a proton off of the carbon attached to the Ph3P group (triphenylphosphine) group. This creates the nucleophile, otherwise known as
an ylide
In a Wittig reaction, a nucleophilic ylide is formed which will attack
the partially positive carbon in a C=O bond
