Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Organic Chemistry II Lab Final
front 1 What is the electrophile in a Friedel-Crafts reaction? | back 1 carbocation |
front 2 How is the alkyl electrophile formed during Friedel-Crafts alkylation? | back 2 Lewis acid catalyst is used to remove the halogen from an alkyl halide, creating a cation which is then attacked by the pi electrons in the aromatic system. |
front 3 How is the aromatic ring reformed during the electrophilic aromatic substitution using Friedel-Crafts alkylation and acylation? | back 3 water or the conjugate base of the acid catalyst attacks the hydrogen on the same carbon as the substituent being added, causing the electrons to fall back into the ring |
front 4
These are all drawbacks of: | back 4 Friedel-Crafts alkylation |
front 5 CN, HOSO2, NO2 act as what kind of substituents on a benzene ring? What effect does this have on substituents being added to the ring? Are halogens considered part of this group? | back 5 deactivating, withdraws electron density from ortho positions causing the substituents to direct to the meta position preferentially, halogens are considered deactivating bc they are slower than benzene but you can perform Friedel-Crafts on a halobenzene and it will direct ortho-para. |
front 6 What can cause a substituent to prefer the para position over the ortho position? | back 6 steric hindrance |
front 7 In the nitration of methyl benzoate, how is the nitronium ion intermediate formed? | back 7 nitric acid is protonated by the sulfuric acid catalyst, which causes the formation of a small, stable leaving group: water |
front 8 What effect does temperature have on nitration products? | back 8 an increase in temperature correlates to an increased number of additions to a benzene ring. To obtain a monosubstituted product, the reaction must take place at cold temperatures. |
front 9 What is the relationship between borneol and isoborneol? | back 9 diastereomers |
front 10 How is camphor able to form two stereoisomers? | back 10 planar geometry of the C=O double bond allows it to be attacked from above and below |
front 11 A bottom-side attack of camphor using sodium borohydride will cause the ___________ isomer to form. | back 11 Isoborneol |
front 12 A top-face attack of camphor using sodium borohydride will cause the ___________ isomer to form. | back 12 Borneol |
front 13 Isoborneol is converted to camphor by _____________ using sodium hypochlorite. | back 13 oxidation |
front 14 What mechanism is used to oxidize isoborneol to camphor? | back 14 E2 elimination |
front 15 How many chiral centers do isoborneol and borneol possess respectively? | back 15 3 |
front 16 What is the chiral conformation of borneol? | back 16 R,S |
front 17 What is the chiral conformation of isoborneol? | back 17 R,R |
front 18 In order to perform the oxidation of camphor using household bleach, what was done to sodium hypochlorite (NaOCl)? | back 18 it was converted to the oxidizing agent hypochlorous acid using glacial acetic acid |
front 19 What is considered the nucleophile in a Grignard reagent | back 19 the carbon attached to the magnesium |
front 20 Nucleophiles are Lewis _________. | back 20 bases |
front 21 What does the carbon nucleophile in a Grignard reagent attack? | back 21 the partially positive carbon in the C=O bond |
front 22 Why is it so important to perform a Grignard Reaction using anhydrous conditions? | back 22 carbon nucleophiles are aggressive and will react with atmospheric moisture to form MgBrOH |
front 23 The purpose of HCl in Grignard reactions is to | back 23 protonate the oxygen on the product to form an alcohol |
front 24 What test is performed to detect the presence of an aldehyde? | back 24 Tollen's test |
front 25 What test is performed to detect the presence of a methyl ketone? | back 25 Iodoform test |
front 26 What aldehyde will produce a positive Iodoform? | back 26 ethanal, also known as acetaldehyde |
front 27 The iodoform reaction proceeds via | back 27 alpha elimination |
front 28 What reaction uses: Ag(NH3)2 under basic conditions? | back 28 Tollen's test |
front 29 In Iodoform reactions, what does iodine substitute? | back 29 all 3 alpha hydrogens on the methyl group |
front 30 What does a positive Iodoform test produce? | back 30 bright yellow precipitate |
front 31 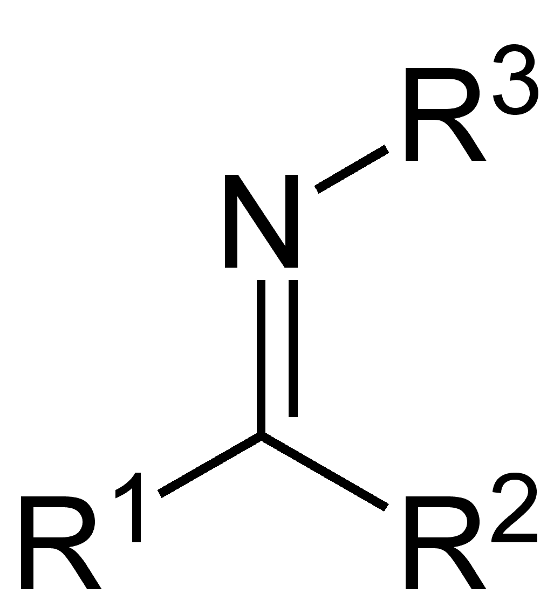 What is this compound called? | back 31 imine |
front 32 What is the function of the acid catalyst in imine formation? | back 32 the elimination of water to form the double bond between carbon and nitrogen. |
front 33 What happens if you use NHO3 and H2SO4 on an aniline ring? | back 33 the lone pair on the NH2 group is a Lewis base and will react with the Lewis acid catalyst |
front 34 True or False: it is ok to add boiling chips to a round bottom flask that is already being heated | back 34 False |
front 35 True or False: If a chemical is splashed on your face, remove your goggles before washing | back 35 False |
front 36 What is the purpose of reflux? | back 36 allows the reaction to be heated at the boiling point of the reactants which increases the overall rate of the ration without loss of product from evaporation, potentially increasing the overall yield |
front 37 What is the purpose of distillation? | back 37 uses a condenser to collect and isolate a pure product from a mixture of reactants based on differences in boiling points |
front 38 What is the purpose of reacting an aldehyde/ketone with semicarbazone or 2,4-DNP? | back 38 convert liquid aldehydes and ketones to a solid for the purposes of melting point comparison |
front 39 Semicarbazide possesses two -NH2 groups, one that is alpha to the carbonyl carbon (C=O) and one that is beta. Why is the alpha group less reactive? | back 39 electrons are tied up in resonance with the carbonyl group |
front 40 Why is the carbanion formed during aldol condensation stable? | back 40 resonance structure called an enolate |
front 41 What reagent is used to form the enolate during aldol condensation and what does it attack? | back 41 NaOH, alpha hydrogens |
front 42 Why do aldol products that form from aromatic aldehydes/ketones spontaneously dehydrate? | back 42 because they are conjugated and extremely stable |
front 43 How do you avoid creating the Cannizarro side product that is sometimes formed during an aldol condensation? | back 43 combine the reactants together before adding base so that benzaldehyde does not react with it |
front 44 What does the excess benzaldehyde react with during a Cannizarro side product formation of an aldol condensation, and what does it form? | back 44 NaOH, benzoic acid and benzyl alcohol |
front 45 What is Fisher Esterification? | back 45 the acid-catalyzed and reversible reaction between a carboxylic acid and an alcohol to create an ester |
front 46 According to LeChatelier's Principle, how would you tip the reversible Fisher Esterification reaction toward the formation of product? | back 46 removal of product as it is formed, heating the solution, and increasing the concentration of the reactants |
front 47 What is the role of acetic acid and sulfuric acid in Fischer Esterification? | back 47 acetic acid is the carboxylic acid and sulfuric acid is the catalyst. When combined they form a carbocation that will react with alcohol. Sulfuric acid also serves to remove the water byproduct, shifting the equilibrium in favor of the products |
front 48 What is the purpose of washing the Fisher Esterification product in sodium bicarbonate? | back 48 its a weak base which will quench any excess acid to its conjugate base (a salt) which will precipitate out as a solid and can be easily removed |
front 49 What can you calculate using this equation? [(2xC+2)+N-H-X] / 2 | back 49 degrees of unsaturation |
front 50 What kind of spectroscopy can be used to determine functional groups? | back 50 IR spectroscopy |
front 51 The number of signals (peaks) in Proton-NMR is equivalent to the number of individual or sets of | back 51 unique hydrogens |
front 52 The __________ reflects the number of H's associated with a particular peak | back 52 integration |
front 53 Ppm, or parts per million, corresponds with bond polarity. It is also called | back 53 chemical shift |
front 54 What will have a Carbon NMR peak in the 200 ppm range? | back 54 C=O |
front 55 What is generally considered the aliphatic range for Carbon NMR? | back 55 20-140 ppm |
front 56 What is generally considered the aromatic range for Carbon NMR? | back 56 110-170 ppm |
front 57 What is generally considered the C-X or C-O range for Carbon NMR? | back 57 45-90 ppm |
front 58 What is generally considered the COOH range for Proton NMR? | back 58 9.8-13 ppm |
front 59 What is generally considered the aldehyde range for Proton NMR? | back 59 9-10 ppm |
front 60 What is generally considered the aromatic range for Proton NMR? | back 60 6.5-8.5 ppm |
front 61 What is generally considered the amine range for Proton NMR? | back 61 0.7-2.7 ppm |
front 62 What is generally considered the ~OH range for Proton NMR? | back 62 1-5 ppm |
front 63 What is generally considered the C-O,C-X,C-NR2 range for Proton NMR? | back 63 2.5-4.3 ppm |
front 64 What is generally considered the amide range for Proton NMR? | back 64 5-8 ppm |
front 65 What is generally considered the alkene range for Proton NMR? | back 65 4.4-7 ppm |
front 66 What is generally considered the ketone range for Proton NMR? | back 66 2-3 ppm |
front 67 What groups will have the highest Proton NMR chemical shifts? | back 67 carboxylic acids and aldehydes |
front 68 What 3 clues can Proton NMR give about the structure of an unknown? | back 68 chemical shift provides functional group clues, N+1 (splitting pattern) can give connectivity clues, and integration can determine the number of unique hydrogens |
front 69 How many degrees of unsaturation does an aromatic ring have? | back 69 4 |
front 70 What type of functional group will have an IR peak at 1700? | back 70 C=O |
front 71 Where will aromatic ring peaks show up on IR? | back 71 1500-1600 |
front 72 Where will N-H (amine) groups show up on IR? | back 72 3300-3500 |
front 73 An aldehyde will have twin peaks at ______ and ______ and a long, stretched out peak at ______. | back 73 2700, 2800, 1700 |
front 74 C-H bonds in aromatic rings will have IR peaks around | back 74 3000-3100 |
front 75 Where will nitriles (C triple bonded to N) show up on IR? | back 75 2200 |
front 76 What is considered the aliphatic range of Proton NMR? | back 76 1-4.5 ppm |
front 77 What is considered the aromatic range of Proton NMR? | back 77 6.5-8.5 ppm |
front 78 A Wittig reaction uses a strong base (such as NaOH) to pull a proton off of the carbon attached to the Ph3P group (triphenylphosphine) group. This creates the nucleophile, otherwise known as | back 78 an ylide |
front 79 In a Wittig reaction, a nucleophilic ylide is formed which will attack | back 79 the partially positive carbon in a C=O bond |