Which term most precisely describes the cellular process of breaking down large molecules into smaller ones?
Catabolism
Which metabolic process can occur without a net influx of energy from some other process?
Cellular Respiration
Catabolic Pathway
A metabolic pathway that releases energy by breaking down complex molecules to simpler molecules.
Catabolism is to anabolism, as ______ is to ______.
A) exergonic; spontaneous
B) exergonic; endergonic
C) free energy, entropy
D) work; energy
E) entropy, enthalpy
exergonic; endergonic
Anabolic Pathway
A metabolic pathway that consumes energy to synthesize a complex molecule from simpler molecules.
Kinetic Energy
Energy can be associated with the relative motion of object.
Thermal Energy
Kinetic energy associated with the random movement of atoms or molecules.
Heat
Thermal energy transferred from one object to another.
Potential Energy
Energy that matter possesses because of its location or structure.
Chemical Energy
The potential energy available for release in a chemical reaction.
First Law of Thermodynamics
Energy can be transferred or transformed but neither can be created or destroyed.
Second Law of Thermodynamics
Every Energy transfer or transformation increases the disorder (entropy) of the universe.
Thermodynamics
The study of energy transformation that occur in a collection of matter.
Entropy
A thermodynamic quantity representing the unavailability of a system's thermal energy for conversion into mechanical work, often interpreted as the degree of disorder or randomness in the system.
Spontaneous Process
The time-evolution of a system in which it releases free energy (usually as heat) and moves to a lower, more thermodynamically stable energy state.
Consume energy to build up polymers from monomers
Anabolic Pathways
Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the Second Law of Thermodynamics?
As a consequence, growing organisms cause a greater increase in entropy in their environment than than the decrease in entropy associated with their growth.
Whenever energy is transformed, there is always an increase in the...
entropy of the universe
Second Law of Thermodynamics
Every chemical reaction must increase the total entropy of the universe
Type of reaction that would decrease the entropy within a cell
Anabolic Reaction
What is an example of potential energy rather than kinetic?
A molecule of glucose
Metabolism consists of all the energy transformation reactions in an organism.
True
The energy in a molecule* that is available for conversion to some other form. All molecules* have an inherent free energy.
Free energy
The difference in free energy between two molecules or “states.”
Free energy change (DG)
A+B→C+D
Example: glucose + fructose --> sucrose and H2O.
Free energy change
If DG < 0, chemical reaction is
Exergonic
If DG > 0, chemical reaction is
Endergonic
Requires input of energy. Cannot occur unless energy from some other source is added.
Endergonic
Cells carry out endergonic reactions by _______ them to a more strongly exergonic reaction.
Coupling
What is the most common exergonic reaction
ATP hydrolysis
An exergonic reaction or process is used to drive an endergonic reaction or process.
Energy coupling
Typical nucleotide with 3 phosphates attached
ATP (Adenosine Triphosphate)
Terminal phosphate is removed, producing ADP and Pi (inorganic phosphate)
ATP hydrolysis
- Overcoming a barrier to start an exergonic reaction
- Energy must be added to break bonds before others are formed.
- A common non‐catalytic way to overcome activation energy (EA) is heat
Activation energy
- A substance that accelerates a chemical reaction without being changed by it.
- Act by lowering the activation energy, so that it is not a barrier under cellular conditions.
Catalyst
- Accelerate the rates of exergonic chemical reactions by lowering the activation energy.
- Cannot catalyze endergonic reactions
Ezyme
How is cellular metabolism regulated?
Enzymes
- All biochemical reactions are exergonic.
- Most exergonic reactions require catalysis to overcome the activation energy.
True
Breakdown of glucose to make ATP
Catabolic
Synthesis of macromolecules from smaller subunits.
Anabolic
Usually proteins
Enzymes
Cavity where chemical reaction takes place
Active site
Change in protein folding tightens fit around substrate after binding
Induced fit
- substrate(s) bind in active site
- enzyme undergoes slight change in 3D structure (induced fit)
- chemical reaction takes place
- product(s) released
Steps in catalysis
Enzymes lower the activation energy by:
- acting as a template to bring reactive groups into proximity
- straining bonds & stabilizing transition state
- providing microenvironment to chemical reaction
- participating directly in chemical reaction
Cofactor
Non‐protein molecule required for catalysis
Coenzyme
Organic molecule that acts as cofactor (subset of “cofactor”)
Competitive inhibition
A molecule that is similar in shape to the substrate binds to and blocks the active site.
Noncompetitive regulation
Molecule binds to a site other than the active site and inhibits or promotes catalysis
Allosteric site
Site to which regulator binds. Not the active site.
Binding of a molecule...
induces a change in conformation. Change in conformation changes shape of active site.
Conformations
Proteins exist in 2 or more alternative, slightly different 3D shapes
Can activate (upper) or be inhibitory (lower).
Allosteric regulation
Cooperativity
Occurs in enzymes w/ more than one subunit. Binding of one substrate molecule increases the stability of the active conformation.
Feedback inhibition
A “downstream” product inhibits an earlier step in a biochemical pathway.
Phosphorylated Intermediate
The recipient molecule with the phosphate group covalently bonded to it
Substrate
The reactant an enzyme acts on
The enzyme binds to its substrate (or substrates, when there are two or more reactants), forming a(n)
Enzyme-substrate complex
As the substrate enters the active site...
The enzyme changes shape slightly due to interactions between the substrate's chemical groups and the chemical groups on the side chains of the amino acids hat form the active site.
Noncompetitive inhibitors
Do not directly compete with the substrate to bind to the enzyme at the active site.
In cells, what is usually the immediate source of energy for an endergonic reaction?
ATP
What is the fate of the phosphate group that is removed when ATP is converted to ADP?
It is acquired by a reactant in an endergonic reaction.
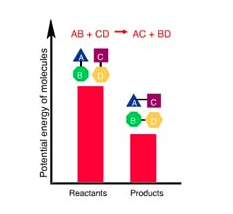
In this reaction _____.
the products have less potential energy than the reactants
How does an enzyme increase the rate of the chemical reaction it catalyzes?
An enzyme reduces the free energy of activation (EA) of the reaction it catalyzes.
Sucrose is a disaccharide, composed of the monosaccharides glucose and fructose. The hydrolysis of sucrose by the enzyme sucrase results in
breaking the bond between glucose and fructose and forming new bonds from the atoms of water.
Zinc, an essential trace element for most organisms, is present in the active site of the enzyme carboxypeptidase. The zinc most likely functions as a(n)
cofactor necessary for enzyme activity.
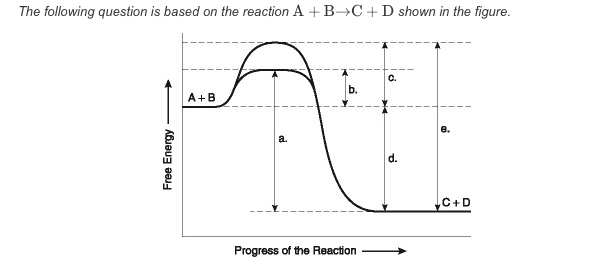
What best describes this reaction?
The amount of free energy released as a result of the catalyzed reaction is indicated by "d."
A series of enzymes catalyze the reaction X→Y→Z→A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
Substance A functions as a(n)...
Allosteric inhibitor
Some of the drugs used to treat HIV patients are competitive inhibitors of the HIV reverse transcriptase enzyme. Unfortunately, the high mutation rate of HIV means that the virus rapidly acquires mutations with amino acid changes that make them resistant to these competitive inhibitors. Where in the reverse transcriptase enzyme would such amino acid changes most likely occur in drug-resistant viruses?
In or near the active site