Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Campbell Biology: Chapter 8
front 1 Which term most precisely describes the cellular process of breaking down large molecules into smaller ones? | back 1 Catabolism |
front 2 Which metabolic process can occur without a net influx of energy from some other process? | back 2 Cellular Respiration |
front 3 Catabolic Pathway | back 3 A metabolic pathway that releases energy by breaking down complex molecules to simpler molecules. |
front 4 Catabolism is to anabolism, as ______ is to ______. A) exergonic; spontaneous B) exergonic; endergonic C) free energy, entropy D) work; energy E) entropy, enthalpy | back 4 exergonic; endergonic |
front 5 Anabolic Pathway | back 5 A metabolic pathway that consumes energy to synthesize a complex molecule from simpler molecules. |
front 6 Kinetic Energy | back 6 Energy can be associated with the relative motion of object. |
front 7 Thermal Energy | back 7 Kinetic energy associated with the random movement of atoms or molecules. |
front 8 Heat | back 8 Thermal energy transferred from one object to another. |
front 9 Potential Energy | back 9 Energy that matter possesses because of its location or structure. |
front 10 Chemical Energy | back 10 The potential energy available for release in a chemical reaction. |
front 11 First Law of Thermodynamics | back 11 Energy can be transferred or transformed but neither can be created or destroyed. |
front 12 Second Law of Thermodynamics | back 12 Every Energy transfer or transformation increases the disorder (entropy) of the universe. |
front 13 Thermodynamics | back 13 The study of energy transformation that occur in a collection of matter. |
front 14 Entropy | back 14 A thermodynamic quantity representing the unavailability of a system's thermal energy for conversion into mechanical work, often interpreted as the degree of disorder or randomness in the system. |
front 15 Spontaneous Process | back 15 The time-evolution of a system in which it releases free energy (usually as heat) and moves to a lower, more thermodynamically stable energy state. |
front 16 Consume energy to build up polymers from monomers | back 16 Anabolic Pathways |
front 17 Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the Second Law of Thermodynamics? | back 17 As a consequence, growing organisms cause a greater increase in entropy in their environment than than the decrease in entropy associated with their growth. |
front 18 Whenever energy is transformed, there is always an increase in the... | back 18 entropy of the universe |
front 19 Second Law of Thermodynamics | back 19 Every chemical reaction must increase the total entropy of the universe |
front 20 Type of reaction that would decrease the entropy within a cell | back 20 Anabolic Reaction |
front 21 What is an example of potential energy rather than kinetic? | back 21 A molecule of glucose |
front 22 Metabolism consists of all the energy transformation reactions in an organism. | back 22 True |
front 23 The energy in a molecule* that is available for conversion to some other form. All molecules* have an inherent free energy. | back 23 Free energy |
front 24 The difference in free energy between two molecules or “states.” | back 24 Free energy change (DG) |
front 25 A+B→C+D Example: glucose + fructose --> sucrose and H2O. | back 25 Free energy change |
front 26 If DG < 0, chemical reaction is | back 26 Exergonic |
front 27 If DG > 0, chemical reaction is | back 27 Endergonic |
front 28 Requires input of energy. Cannot occur unless energy from some other source is added. | back 28 Endergonic |
front 29 Cells carry out endergonic reactions by _______ them to a more strongly exergonic reaction. | back 29 Coupling |
front 30 What is the most common exergonic reaction | back 30 ATP hydrolysis |
front 31 An exergonic reaction or process is used to drive an endergonic reaction or process. | back 31 Energy coupling |
front 32 Typical nucleotide with 3 phosphates attached | back 32 ATP (Adenosine Triphosphate) |
front 33 Terminal phosphate is removed, producing ADP and Pi (inorganic phosphate) | back 33 ATP hydrolysis |
front 34
| back 34 Activation energy |
front 35
| back 35 Catalyst |
front 36
| back 36 Ezyme |
front 37 How is cellular metabolism regulated? | back 37 Enzymes |
front 38
| back 38 True |
front 39 Breakdown of glucose to make ATP | back 39 Catabolic |
front 40 Synthesis of macromolecules from smaller subunits. | back 40 Anabolic |
front 41 Usually proteins | back 41 Enzymes |
front 42 Cavity where chemical reaction takes place | back 42 Active site |
front 43 Change in protein folding tightens fit around substrate after binding | back 43 Induced fit |
front 44
| back 44 Steps in catalysis |
front 45 Enzymes lower the activation energy by: | back 45
|
front 46 Cofactor | back 46 Non‐protein molecule required for catalysis |
front 47 Coenzyme | back 47 Organic molecule that acts as cofactor (subset of “cofactor”) |
front 48 Competitive inhibition | back 48 A molecule that is similar in shape to the substrate binds to and blocks the active site. |
front 49 Noncompetitive regulation | back 49 Molecule binds to a site other than the active site and inhibits or promotes catalysis |
front 50 Allosteric site | back 50 Site to which regulator binds. Not the active site. |
front 51 Binding of a molecule... | back 51 induces a change in conformation. Change in conformation changes shape of active site. |
front 52 Conformations | back 52 Proteins exist in 2 or more alternative, slightly different 3D shapes |
front 53 Can activate (upper) or be inhibitory (lower). | back 53 Allosteric regulation |
front 54 Cooperativity | back 54 Occurs in enzymes w/ more than one subunit. Binding of one substrate molecule increases the stability of the active conformation. |
front 55 Feedback inhibition | back 55 A “downstream” product inhibits an earlier step in a biochemical pathway. |
front 56 Phosphorylated Intermediate | back 56 The recipient molecule with the phosphate group covalently bonded to it |
front 57 Substrate | back 57 The reactant an enzyme acts on |
front 58 The enzyme binds to its substrate (or substrates, when there are two or more reactants), forming a(n) | back 58 Enzyme-substrate complex |
front 59 As the substrate enters the active site... | back 59 The enzyme changes shape slightly due to interactions between the substrate's chemical groups and the chemical groups on the side chains of the amino acids hat form the active site. |
front 60 Noncompetitive inhibitors | back 60 Do not directly compete with the substrate to bind to the enzyme at the active site. |
front 61 In cells, what is usually the immediate source of energy for an endergonic reaction? | back 61 ATP |
front 62 What is the fate of the phosphate group that is removed when ATP is converted to ADP? | back 62 It is acquired by a reactant in an endergonic reaction. |
front 63 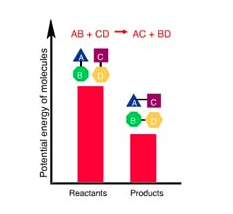 In this reaction _____. | back 63 the products have less potential energy than the reactants |
front 64 How does an enzyme increase the rate of the chemical reaction it catalyzes? | back 64 An enzyme reduces the free energy of activation (EA) of the reaction it catalyzes. |
front 65 Sucrose is a disaccharide, composed of the monosaccharides glucose and fructose. The hydrolysis of sucrose by the enzyme sucrase results in | back 65 breaking the bond between glucose and fructose and forming new bonds from the atoms of water. |
front 66 Zinc, an essential trace element for most organisms, is present in the active site of the enzyme carboxypeptidase. The zinc most likely functions as a(n) | back 66 cofactor necessary for enzyme activity. |
front 67 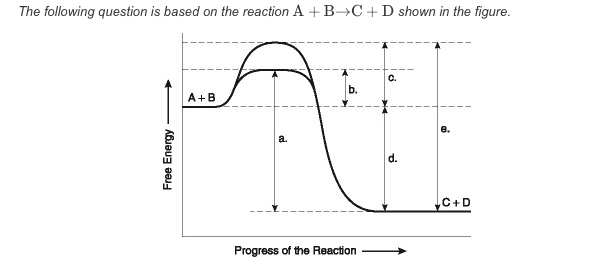 What best describes this reaction? | back 67 The amount of free energy released as a result of the catalyzed reaction is indicated by "d." |
front 68 A series of enzymes catalyze the reaction X→Y→Z→A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme. Substance A functions as a(n)... | back 68 Allosteric inhibitor |
front 69 Some of the drugs used to treat HIV patients are competitive inhibitors of the HIV reverse transcriptase enzyme. Unfortunately, the high mutation rate of HIV means that the virus rapidly acquires mutations with amino acid changes that make them resistant to these competitive inhibitors. Where in the reverse transcriptase enzyme would such amino acid changes most likely occur in drug-resistant viruses? | back 69 In or near the active site |