What is meant by the term bumping?
Sudden and uncontrolled boiling of liquid
What is the best technique for removing a round bottom flask from an oil bath?
Wearing heat-resistant gloves, raise the clamp to lift the flask out of the oil bath. Allow the flask to cool for a while, then use a paper towel to wipe any oil from the bottom of the flask.
Identify items that can be used to control the boiling when heating liquid in a round bottom flask.
A stir bar and stir plate
Boiling chips or stones
According to Markovnikov's rule of the electrophilic addition to an alkene, the electrophile, usually a proton, is more likely to add to the ______ in a double bond. This arrangement places the intermediate carbocation on the _______, which stabilizes it with the presence of ______.
In the major product of a reaction following Markovnikov's rule, the _______ will then end up on the more-substituted carbon in a double bond.
less-substituted carbon
more-substituted carbon
more substituents
neutrophile
Determine whether each alcohol could be a major product of the acid-catalyzed hydration of an alkene.
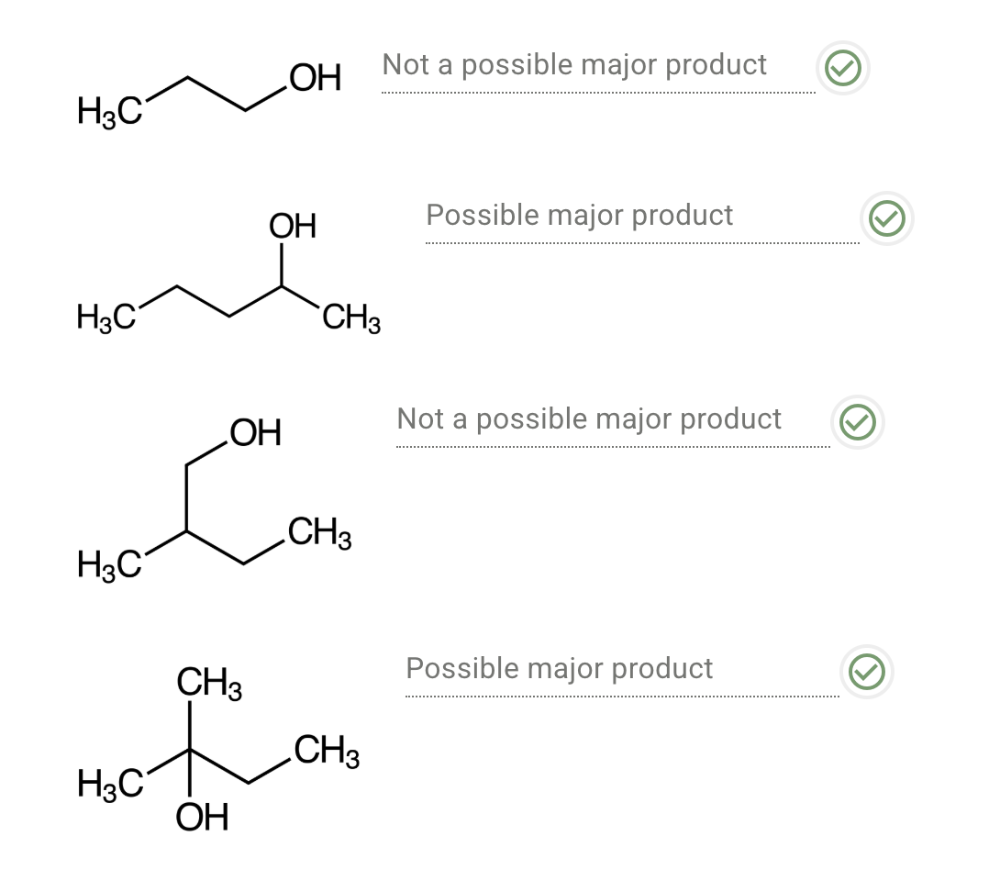
Determine the order of steps in the mechanism of acid-catalyzed hydration of propene.
First step
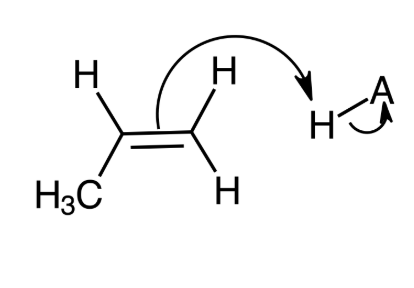
Determine the order of steps in the mechanism of acid-catalyzed hydration of propene.
Second step
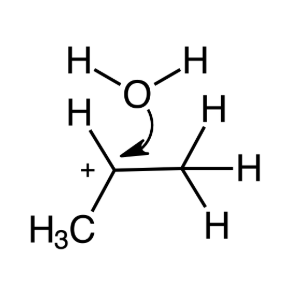
Determine the order of steps in the mechanism of acid-catalyzed hydration of propene.
Third step
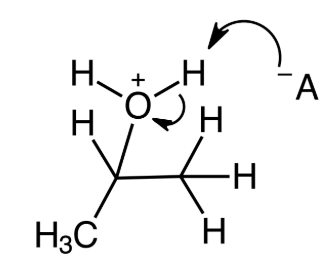
In general, what are the possible products of an acid-catalyzed hydration of an alkene?
Primary alcohol
secondary alcohol
tertiary alcohol
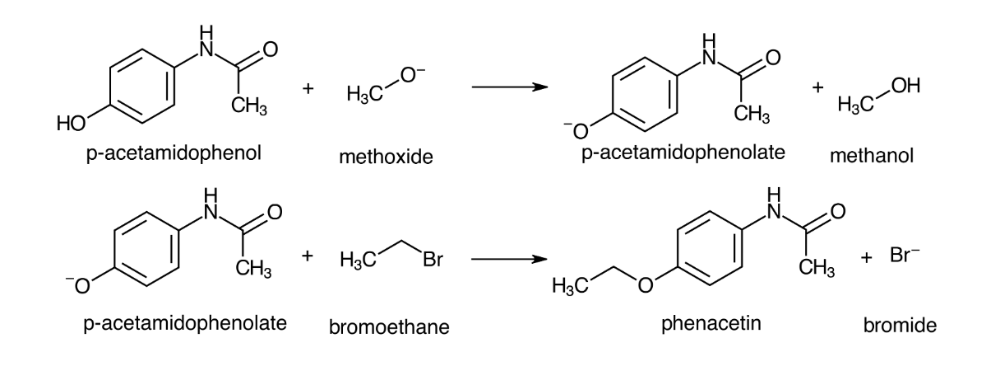
Consider the mechanism of forming phenacetin from p-acetaminophenol, bromoethane, and methoxide.
Identify the species that performs each listed role in the reaction.
Acid____
Base____
Leaving group ____
Nucleophile ____
Acid: p-Acetamindophenol
Base: Methoxide
Leaving group: Bromide
Nucleophile: p-Acetamindophenolate
In Williamson ether synthesis, you start by adding a base like methoxide to deprotonate a phenol so the deprotonated phenolate can act as nucleophile. The moles of base should be ____ the moles of phenol. Less base might result in the phenol ______. More base might result in the base_____.
Equal to
not reacting completely
acting as the nucleophile
The SN2 mechanism occurs in _____ with the nucleophile attacking _____ the leaving group leaves. Therefore, unsubstituted electrophiles react _____ than substituted electrophiles due to the ______.
one step, at the same time
Faster, decreased crowding
During a recrystallization, the goal is to form purified crystals of a solid out of solution. However, you might form an oil instead.
How should you respond if an oil forms during your recrystallization?
Add more of the recrystallization solvent to form a solution before cooling again
What is the recommended order of measurements to report the most accurate melting point possible?
Use slow heating to carefully observe melting_____
Use slow heating to confirm the careful measurement_____
Use quick heating to estimate the melting point_____
second step
third step
first step
What characteristics should a good sample for melting point determination have?
Solid phase
small particles
thoroughly dry
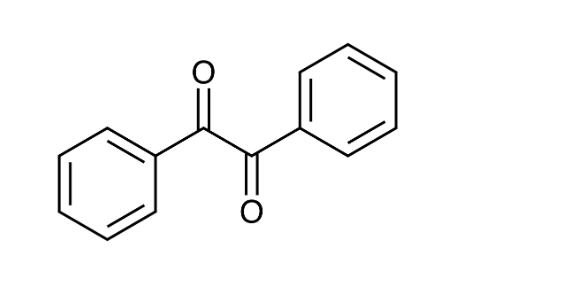
Consider the structure of benzil and its reduction.
The functional group reduced in benzil is ___.
An appropriate reducing agent for this reaction is _____
a ketone
sodium borohydride
Is often the ending point of an arrow in a mechanism____
proton
Is often the starting point of an arrow in a mechanism____
hydride
Can act as a nucleophile
hydride
Can act as an electrophile
proton
Can act as a reducing agent
hydride
Can act as an oxidizing agent
proton
Suppose you notice a solid impurity in a small liquid sample with a volume less than 10 mL.
What technique can you use to remove the solid impurity?
Filter the sample through a Pasteur pipet with glass wool in it
What statements about the possible hazards of sodium borohydride are correct?
Sodium borohydride is corrosive.
Sodium borohydride is flammable.
Sodium borohydride can have a violent reaction with acids