lowest possible temperature
there is a lowest possible temperature (−273 °C),known as absolute zero, where the particles have least kinetic energy
Convert temperatures between kelvin and degrees Celsius
Kelvin = x degrees celsius + 273
evidence for the kinetic particle model of matter
The random, zig-zag motion of visible particles (like pollen grains or smoke particles) suspended in a fluid, known as Brownian motion, provides evidence for the kinetic particle model of matter. This motion occurs because the large, suspended particles are bombarded from random directions by smaller, faster-moving, lighter invisible gas or liquid molecules, which transfer momentum and cause the visible particles to move unpredictably.
Relationship between pressure and volume
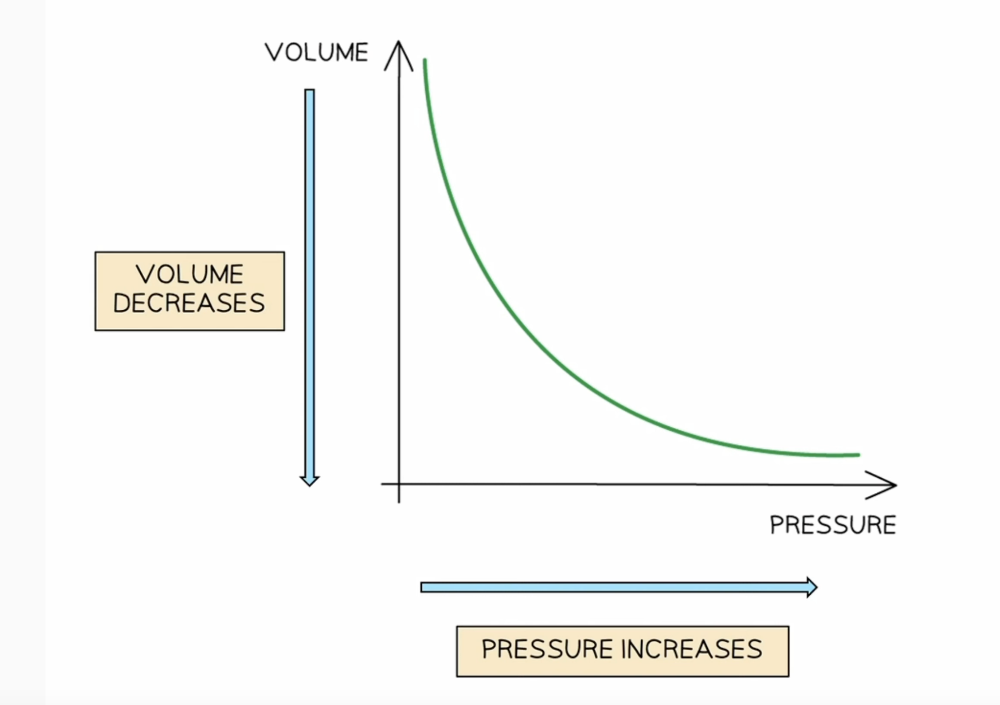
Boyles Law
Pressure x Volume = constant or P1V1=P2V2
*for a fixed mass of gas at constant temperature
They are inversely proportional as pressure increases volume decreases by the same amount.
Applications and consequences of thermal expansion
- Useful applications of thermal expansion include:
- Liquid-in-glass thermometers
- Temperature-activated switches : utilise a bimetallic strip. It consists of two metals that expand at different rates and bends by a predictable amount at a given temperature.
- Jar Lids:Running hot water over a metal jar lid causes it to expand more than the glass jar, making it easier to open.
- Electrical Cables:Cables are installed with some slack to allow for contraction in cold weather, preventing them from snapping.
Consequences of thermal expansion
- The expansion of solid materials can cause them to buckle if they get too hot
- This could include:
- Metal railway tracks
- Road surfaces
- Bridges
- Objects that are prone to buckling in this way have gaps built in to creates space for the expansion to happen without causing damage
Define specific heat capacity, recall and use the equation
The energy required per unit mass per unit temperature increase.
Specific heat capacity (J/Kg) = change in thermal energy (J) / mass (kg) x change in temperature
Describe experiments to measure the specific heat capacity of a solid and a liquid
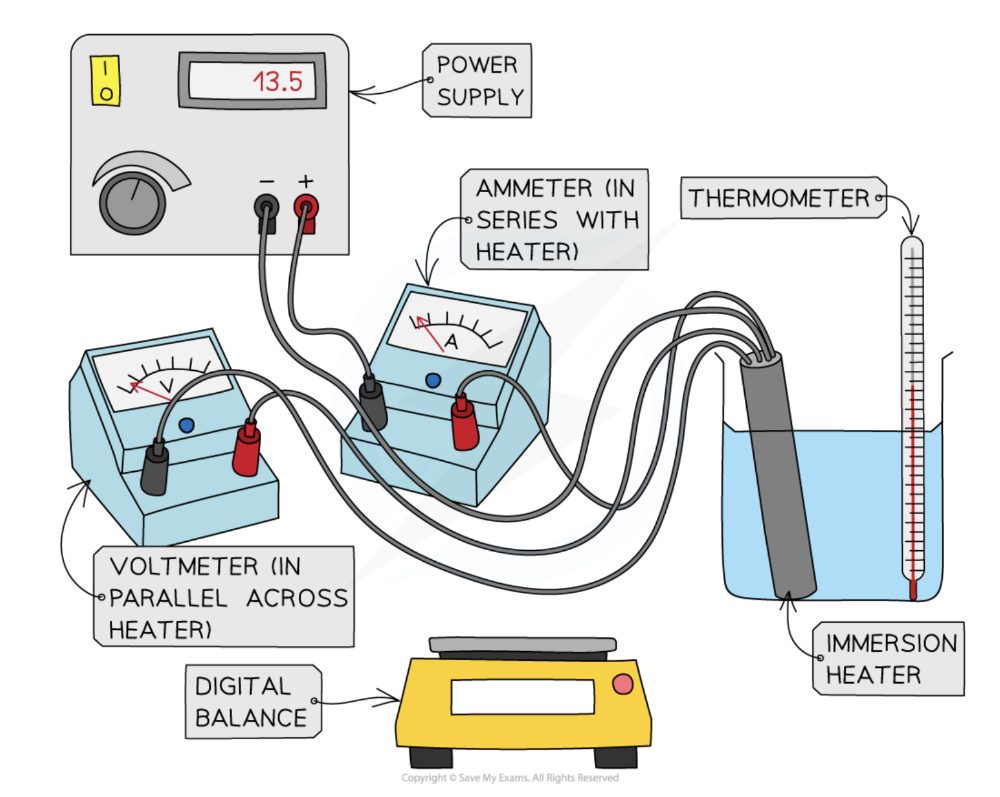
- Place the beaker on the digital balance and press 'zero'
- Add approximately 250 ml of water and record the mass of the water using the digital balance
- Place the immersion heater and thermometer in the water
- Connect up the circuit as shown in the diagram, with the ammeter in series with the power supply and immersion heater, and the voltmeter in parallel with the immersion heater
- Record the initial temperature of the water at time 0 s
- Turn on the power supply, set it at approximately 10 V, and start the stopwatch
- Record the voltage from the voltmeter and the current from the ammeter
- Continue to record the temperature, voltage and current every 60 seconds for 10 minutes
- Repeat steps 2-8, replacing the beaker of water for the solid block of aluminium and starting with recording its mass using the digital balance.
The thermal energy supplied to the block can be calculated using the equation:
- E = Current x voltage x time
- Change in energy = mass x specific heat capacity x change in temperature or current x voltage x time spent heating
- Sub into specific heat capacity equation to find it = Specific heat capacity (J/Kg) = change in thermal energy (J) / mass (kg) x change in temperature
Describe melting and boiling definition
Describe condensation and solidification in terms of particles
Describe evaporation
Describe the differences between boiling and evaporation
Explain evaporation and cooling
Describe melting and boiling : energy input without a change in temperature
Condensation occurs when gas particles lose energy, causing their kinetic energy to decrease. This reduction in kinetic energy allows the intermolecular forces of attraction to pull the particles closer together, forming a liquid.Solidification, or freezing, occurs when liquid particles lose energy, further reducing their kinetic energy. This allows the particles to form stronger intermolecular bonds and become fixed in a rigid, ordered structure, resulting in a solid.
The escape of more energetic particles from the surface of a liquid.
Boiling occurs throughout the entire volume of a liquid at a specific, constant temperature (the boiling point), where bubbles of vapor form within the liquid.Evaporation, on the other hand, occurs only at the surface of a liquid and can happen at any temperature below the boiling point.
Evaporation causes cooling of a liquid because the most energetic particles are the ones that escape from the surface. This removal of higher-energy particles reduces the average kinetic energy of the remaining particles in the liquid, leading to a decrease in the liquid's temperature. An object in contact with an evaporating liquid experiences cooling because the liquid absorbs thermal energy from the object to facilitate the evaporation process.
Describe experiments to demonstrate the properties of good thermal conductors and bad thermal conductors (thermal insulators)
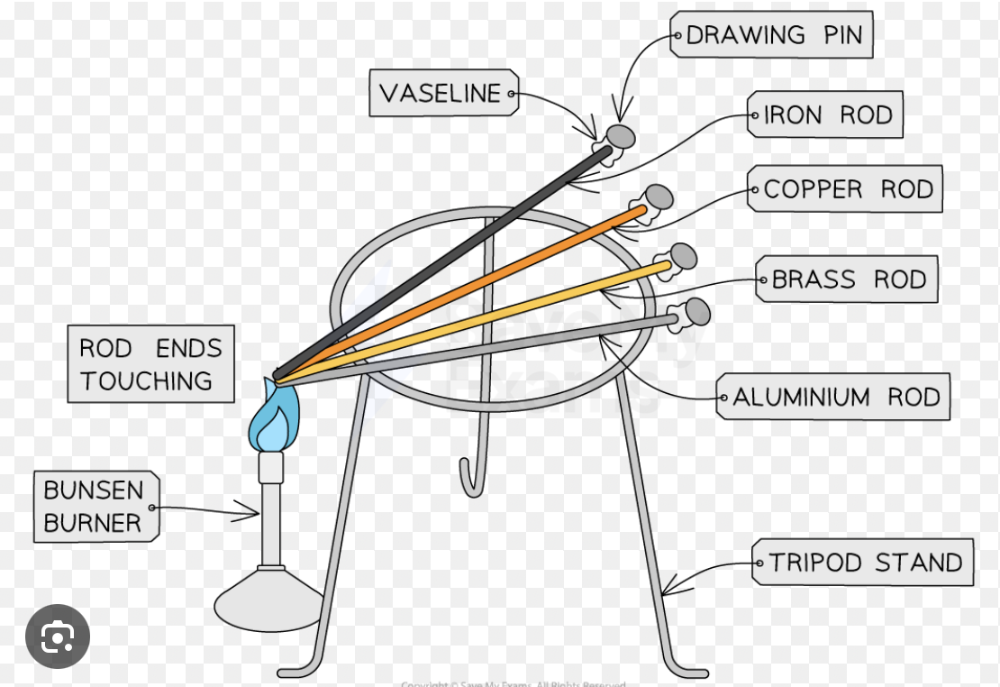
- Take several rods of the same length and cross-sectional area but of different materials (e.g., copper, wood, plastic).
- Attach a drawing pin to the end of each rod using a small amount of wax or vaseline.
- balance the rods on a tripod with all the ends touching with a bunsen burner underneath.
- Start a stopwatch and observe the time it takes for the wax on each rod to melt and the pin to fall off.
- The rod with the shortest time to fall off is the best conductor, while the rod with the longest time is the poorest conductor (an insulator).
- rotate half wood half metal pole wrapped in paper over a bunsen burner. \
- stop when paper is discoloured.
- Remove the rod from the flame, gently unwrap the paper and
observe the burn pattern
- A distinct pattern is seen;
- Where the paper touched the metal surface it is undamaged
- Where the paper touched the wood surface it is charred
- A distinct pattern is seen;
- this is because metal is a good conductor so heat was transferred from the paper into the metal and along the length of the metal. This prevented the paper getting hot Wood is a good insulator, meaning it is a poor conductor of heat. Where the paper touched the wood, heat was not transferred from the paper. This meant that the paper got hot enough to start to burn.
Explain convection in liquids and gases and describe experiments to illustrate convection
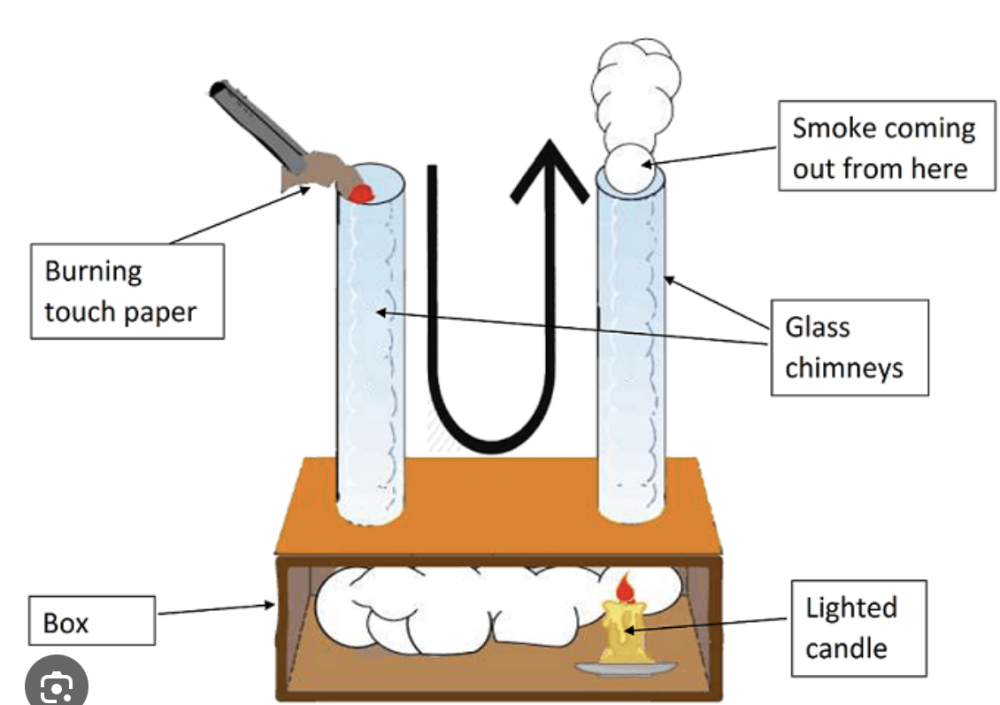
Convection occurs in liquids and gases (fluids) when heat causes the fluid to expand and become less dense, causing it to rise. Cooler, denser fluid then sinks to take its place, creating a continuous cycle called a convection current that transfers thermal energy.
- Setup: Fill a transparent container (like a beaker) with water.
- Procedure: Place a few crystals of potassium permanganate at the bottom of the beaker. Gently heat the beaker from below.
- Observation: You will see a purple column of coloured water rise from the bottom of the beaker as the water is heated. The warmed water, carrying the dissolved crystals, rises, while cooler, denser water sinks to take its place, forming a visible convection current.
- Setup: Use a box with two chimneys and a candle placed under one chimney.
- Procedure: Light the candle and hold a smoking splint over the other chimney.
- Observation: You will notice that the smoke is not only drawn up through the chimney with the candle but also sucked down through the other chimney and into the box. Cooler, denser air from the room is drawn down the second chimney to replace the rising hot air, creating the convection current that pulls the smoke along.
explain global warming
With more greenhouse gases, more of the Earth's outgoing infrared radiation is absorbed and reflected back to the surface.
Describe the effect of surface colour (black or white) and texture (dull or shiny) on the emission, absorption and reflection of infrared radiation
Dull black surfaces are excellent emitters, absorbers, and poor reflectors of infrared radiation,
while light, shiny surfaces are poor emitters, poor absorbers, and good reflectors.
Experiments to Distinguish Good and Bad Emitters of Infrared Radiation
- Apparatus: Leslie cube (a metal cube with different surfaces: matte black, glossy black, white, and silver), hot water, an infrared (IR) detector.
-
Method:
- Fill the Leslie cube with boiling water so all its surfaces are at the same temperature.
- Hold the IR detector at the same distance from each of the four faces.
- Measure the intensity of the infrared radiation emitted from each surface.
Method 2
- Apparatus: Four identical metal plates with different surface coatings (e.g., matte black, glossy black, white, shiny silver), each with a coin stuck to it with a small amount of wax.
-
Method:
- Position the plates a fixed distance in front of a heat source (such as a hot Leslie cube filled with hot water).
- Observe how quickly the wax melts on each coin.
Experiments to Distinguish Good and Bad Absorbers of Infrared Radiation
- Attach a thermometer to the back of four different metal plates of the same dimensions (same width, length and thickness).
- Place them all the same distance from a radiant heater and turn on the heater. After a known amount of time, e.g. 5 minutes, turn the heater off. Record the temperature of each plate. The plate of highest temperature is the best absorber.
2 Explain where more than one type of thermal energy transfer is significant, including:
(a) a fire burning wood or coal
(b) a radiator in a car
A fire uses conduction through the fuel, convection by rising hot gases and air, and significant radiation to heat objects and spread the fire.
A car's radiator relies on conduction from the engine coolant to the metal, then convection to the air and radiation to transfer heat away, to cool the engine.