Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
IGCSE Physics 2 | Matter
front 1 lowest possible temperature | back 1 there is a lowest possible temperature (−273 °C),known as absolute zero, where the particles have least kinetic energy |
front 2 Convert temperatures between kelvin and degrees Celsius | back 2 Kelvin = x degrees celsius + 273 |
front 3 evidence for the kinetic particle model of matter | back 3 The random, zig-zag motion of visible particles (like pollen grains or smoke particles) suspended in a fluid, known as Brownian motion, provides evidence for the kinetic particle model of matter. This motion occurs because the large, suspended particles are bombarded from random directions by smaller, faster-moving, lighter invisible gas or liquid molecules, which transfer momentum and cause the visible particles to move unpredictably. |
front 4 Relationship between pressure and volume | back 4 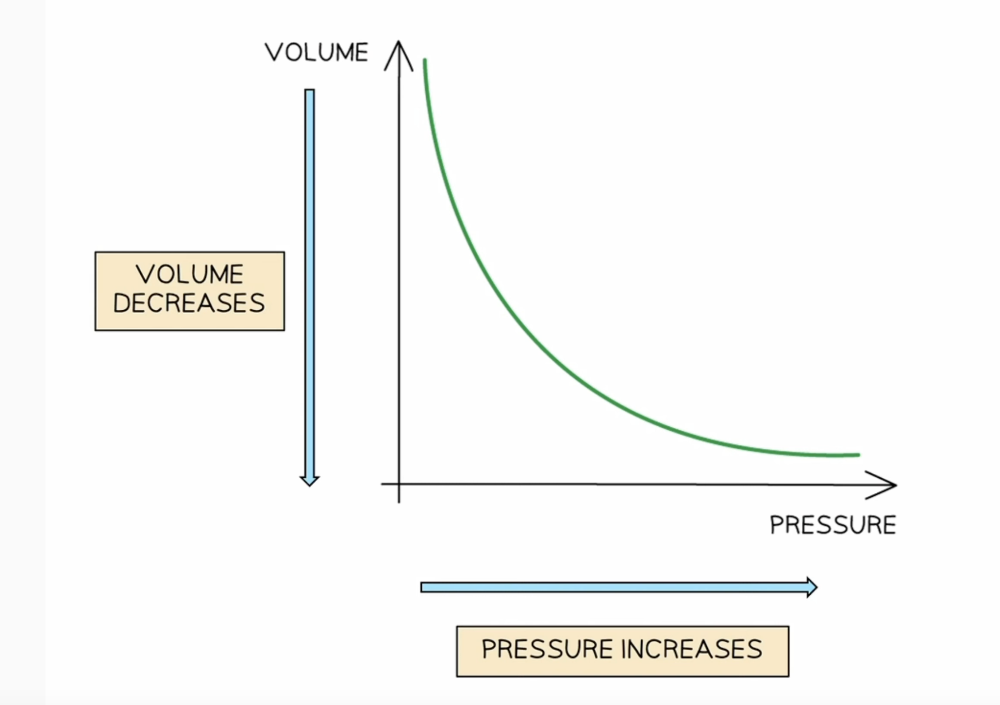 Boyles Law Pressure x Volume = constant or P1V1=P2V2 *for a fixed mass of gas at constant temperature They are inversely proportional as pressure increases volume decreases by the same amount. |
front 5 Applications and consequences of thermal expansion | back 5
Consequences of thermal expansion
|
front 6 Define specific heat capacity, recall and use the equation | back 6 The energy required per unit mass per unit temperature increase. Specific heat capacity (J/Kg) = change in thermal energy (J) / mass (kg) x change in temperature |
front 7 Describe experiments to measure the specific heat capacity of a solid and a liquid | back 7 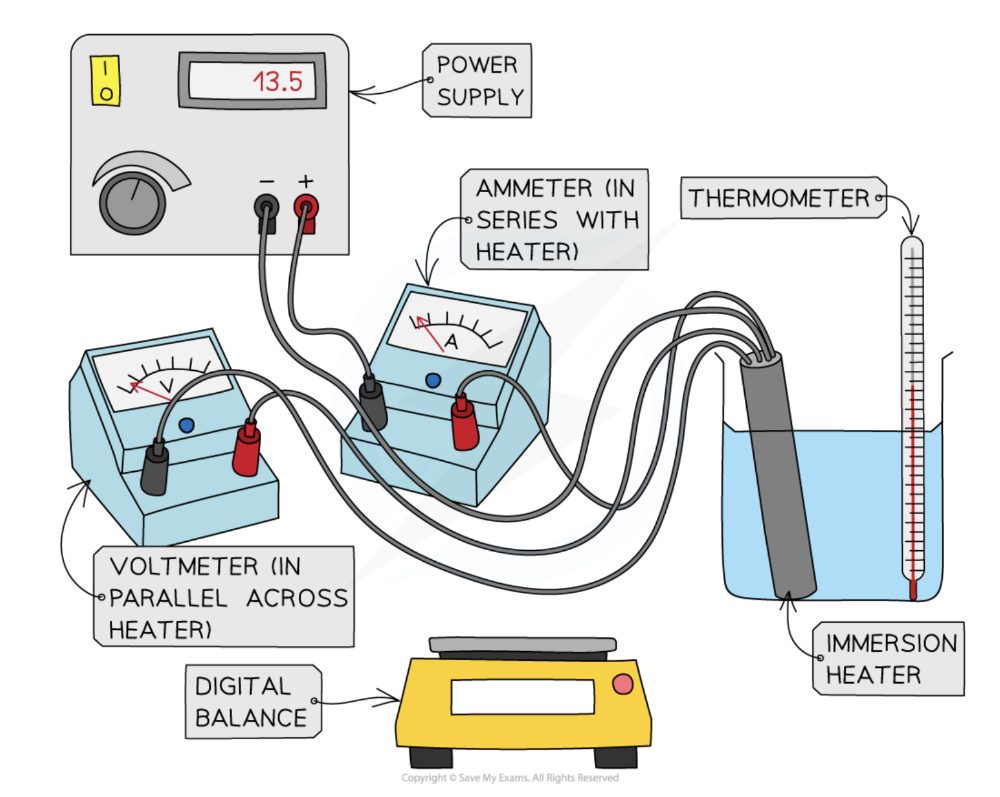
The thermal energy supplied to the block can be calculated using the equation:
|
front 8 Describe melting and boiling definition Describe condensation and solidification in terms of particles Describe evaporation Describe the differences between boiling and evaporation Explain evaporation and cooling | back 8 Describe melting and boiling : energy input without a change in temperature Condensation occurs when gas particles lose energy, causing their kinetic energy to decrease. This reduction in kinetic energy allows the intermolecular forces of attraction to pull the particles closer together, forming a liquid.Solidification, or freezing, occurs when liquid particles lose energy, further reducing their kinetic energy. This allows the particles to form stronger intermolecular bonds and become fixed in a rigid, ordered structure, resulting in a solid. The escape of more energetic particles from the surface of a liquid. Boiling occurs throughout the entire volume of a liquid at a specific, constant temperature (the boiling point), where bubbles of vapor form within the liquid.Evaporation, on the other hand, occurs only at the surface of a liquid and can happen at any temperature below the boiling point. Evaporation causes cooling of a liquid because the most energetic particles are the ones that escape from the surface. This removal of higher-energy particles reduces the average kinetic energy of the remaining particles in the liquid, leading to a decrease in the liquid's temperature. An object in contact with an evaporating liquid experiences cooling because the liquid absorbs thermal energy from the object to facilitate the evaporation process. |
front 9 Describe experiments to demonstrate the properties of good thermal conductors and bad thermal conductors (thermal insulators) | back 9 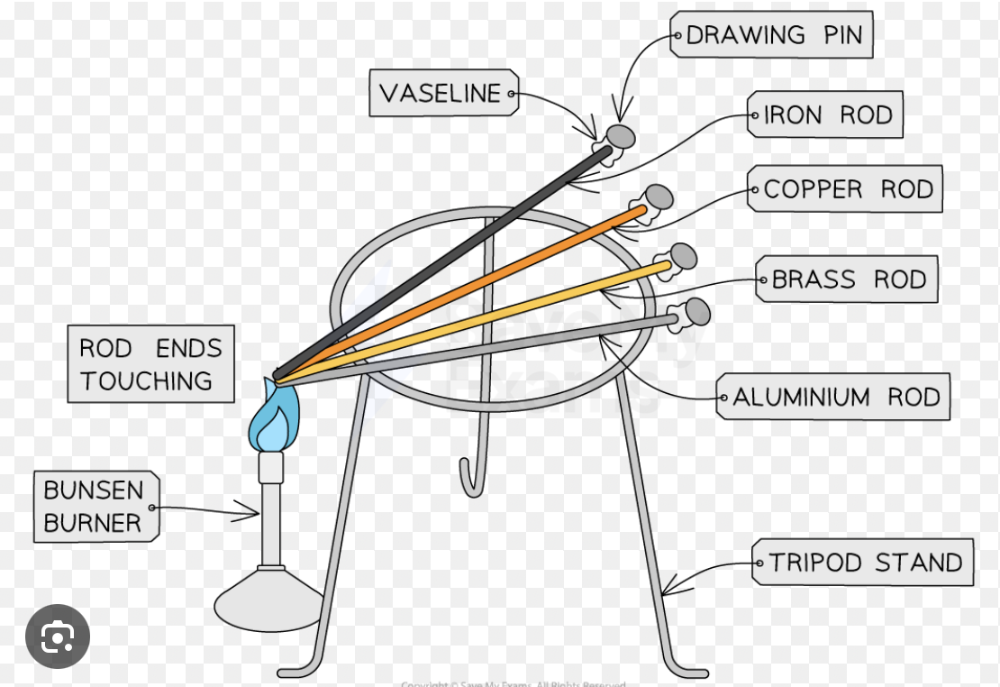
|
front 10 Explain convection in liquids and gases and describe experiments to illustrate convection | back 10 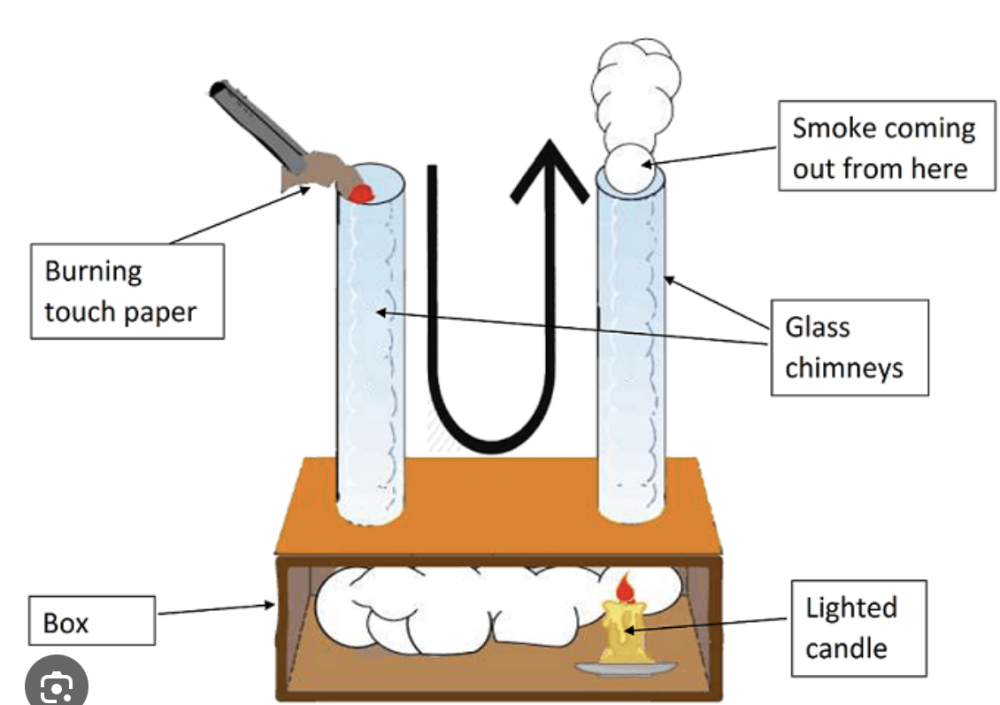 Convection occurs in liquids and gases (fluids) when heat causes the fluid to expand and become less dense, causing it to rise. Cooler, denser fluid then sinks to take its place, creating a continuous cycle called a convection current that transfers thermal energy.
|
front 11 explain global warming | back 11 With more greenhouse gases, more of the Earth's outgoing infrared radiation is absorbed and reflected back to the surface. |
front 12 Describe the effect of surface colour (black or white) and texture (dull or shiny) on the emission, absorption and reflection of infrared radiation | back 12 Dull black surfaces are excellent emitters, absorbers, and poor reflectors of infrared radiation, while light, shiny surfaces are poor emitters, poor absorbers, and good reflectors. |
front 13 Experiments to Distinguish Good and Bad Emitters of Infrared Radiation | back 13
Method 2
|
front 14 Experiments to Distinguish Good and Bad Absorbers of Infrared Radiation | back 14
|
front 15 2 Explain where more than one type of thermal energy transfer is significant, including: (a) a fire burning wood or coal (b) a radiator in a car | back 15 A fire uses conduction through the fuel, convection by rising hot gases and air, and significant radiation to heat objects and spread the fire. A car's radiator relies on conduction from the engine coolant to the metal, then convection to the air and radiation to transfer heat away, to cool the engine. |