Describe the features of the cellular environment (inside and outside).
Intracellular Environment (Inside the Cell)
- Cytoplasm: Gel-like fluid (cytosol + organelles) that fills the cell
- Organelles: Specialized structures like: Nucleus (holds DNA), Mitochondria (energy production), ER & Golgi (protein and lipid processing), Lysosomes (digestion), and Ribosomes (protein synthesis)
- Cytosol: The fluid part of the cytoplasm, containing water, salts, enzymes, and nutrients
- High potassium (K⁺) and low sodium (Na⁺) inside the cell
- Enzymes and proteins carry out essential cell functions
Think of it as a highly organized chemical factory where metabolism and life-sustaining reactions happen.
Extracellular Environment (Outside the Cell)
- Extracellular fluid (ECF): Includes interstitial fluid (between cells) and plasma (in blood) and Provides nutrients , oxygen , and removes waste
- High sodium (Na⁺) and low potassium (K⁺)
- Contains: Hormones and signaling molecules, Structural proteins (like collagen in connective tissues) Immune cells (in some areas)and Acts as a communication and support system for cells
- Think of it as the cell’s supply and messaging network
Interaction Between Inside and Outside
The plasma membrane controls what goes in and out, Transport proteins help move ions, glucose, and other substances and Receptors on the membrane detect signals (e.g., hormones) from the extracellular environment
Explain the processes of diffusion and osmosis and the role they play in membrane transport.
Diffusion is the movement of molecules from an area of high concentration to low concentration until they are evenly spread out.
No energy is required (it’s passive). Occurs with gases, small molecules, and some lipids.
Ex: Oxygen moves from the lungs (high O₂) into the blood (low O₂) by diffusion.
Osmosis is the diffusion of water across a selectively permeable membrane.
- Water moves from an area of low solute concentration (more water) to high solute concentration (less water).
- Goal: balance the concentration of solutes on both sides of the membrane.
Ex: If a cell is in salty water, water moves out of the cell, causing it to shrink.
Role in Membrane Transport: Both diffusion and osmosis are forms of passive transport, meaning the cell does not use energy.
What factors affect the rate of diffusion?
Concentration Gradient Greater difference = faster diffusion
Temperature Higher temperature = faster diffusion (particles move more quickly)
Molecule Size Smaller molecules diffuse faster than larger ones
Membrane PermeabilityIf the membrane is more permeable, diffusion is easier and faster
Surface Area of Membrane Larger surface area = faster diffusion
Distance (Thickness of Membrane) Shorter distance = faster diffusion
Charge (for ions)Opposite charges attract and influence movement; may require channels
Solubility in LipidsLipid-soluble substances (like oxygen or CO₂) diffuse faster through membranes
What is the difference between morality and molality?
Molarity is the number of moles of solute per liter of solution. Depends on volume, which can change with temperature.
Ex: If you dissolve 1 mole of NaCl in enough water to make 1 liter of solution, the molarity is 1 M.
Molality is the number of moles of solute per kilogram of solvent. Depends on mass, which does not change with temperature.
Ex: If you dissolve 1 mole of NaCl in 1 kg of water, the molality is 1 m.
What are two requirements for osmosis to occur?
1. A Selectively Permeable Membrane
The membrane must allow water to pass through, but not solutes (like salt or sugar). This creates a barrier that lets only certain molecules (usually water) move across.
2. A Difference in Solute Concentration on Each Side of the Membrane
There must be an unequal concentration of solutes (e.g., salt or sugar) on the two sides of the membrane. Water moves from the side with lower solute concentration (more water) to the side with higher solute concentration (less water) to try to balance the solute levels.
What is tonicity and how is it relevant clinically?
Tonicity describes how a solution affects the movement of water across a cell membrane by osmosis. It depends on the concentration of non-penetrating solutes (like salt or glucose) outside the cell compared to inside.
Tonicity determines whether a cell will shrink, swell, or stay the same when placed in a solution.
Tonicity is critical in medicine, especially when giving patients IV fluids.
Clinical Examples:
Isotonic IV solution (e.g., 0.9% NaCl)
Used to maintain hydration without changing cell size. Common in trauma, surgery, and general fluid replacement.
Hypotonic IV solution (e.g., 0.45% NaCl)
Used if cells are dehydrated (e.g., in diabetic ketoacidosis). Must be used carefully—can cause cells to swell or burst if overused.
Hypertonic IV solution (e.g., 3% NaCl)
Used to reduce brain swelling or draw fluid out of swollen cells. Can cause cell shrinkage and must be used under close monitoring.
Explain the negative feedback loop responsible for regulating blood osmolality.
Blood osmolality refers to the concentration of solutes (like sodium, glucose, and urea) in the blood. If blood becomes too concentrated (high osmolality) or too dilute (low osmolality), the body uses a negative feedback loop to restore balance and maintain homeostasis.
Describe the characteristics of carrier-mediated transport and explain differences between active and passive forms.
Carrier-mediated transport involves the movement of substances across a cell membrane with the help of carrier proteins embedded in the membrane.
These proteins bind specific molecules, change shape, and move them across the membrane — either with or against the concentration gradient.
The different forms of cell signaling
Autocrine signaling: The cell sends signals to itself.
Ex: Immune cells release signals to amplify their own response.
Paracrine signaling: Signals are sent to nearby cells.
Ex: Neurotransmitters between nerve cells.
Endocrine signaling: Signals (usually hormones) travel through the bloodstream to reach distant target cells.
Ex: Insulin from the pancreas acts on muscles and liver.
Juxtacrine signaling (Direct contact): Cells communicate through physical contact via surface molecules.
Ex: Immune cell recognition and embryonic development.
Synaptic signaling: Specialized paracrine signaling used by neurons; neurotransmitters cross a synapse.
The roles of second messengers and G-proteins
Second messengers are small molecules inside the cell that amplify and carry the signal from a receptor to the target molecules. They are created or released in response to a signal and help trigger a cellular response.
Ex:
- cAMP (cyclic AMP) – activates protein kinases.
- Ca²⁺ (Calcium ions) – involved in muscle contraction, secretion, and more.
- IP₃ (Inositol triphosphate) – releases calcium from internal stores.
- DAG (Diacylglycerol) – works with calcium to activate protein kinase C.
G-proteins (Guanine nucleotide-binding proteins) are molecular switches that help relay signals from receptors (like G protein-coupled receptors or GPCRs) to other proteins inside the cell.
How G-proteins work:
- A signal (like a hormone) binds to a GPCR on the cell membrane.
- The GPCR activates the G-protein by exchanging GDP for GTP.
- The G-protein then splits into two parts, each of which can activate target enzymes or ion channels.
- This leads to the production of second messengers like cAMP or IP₃.
- Eventually, the GTP is hydrolyzed back to GDP, and the G-protein is turned off.
Why are second messengers necessary for certain cell signaling?
Amplification of the Signal: One receptor activation can produce many second messengers, greatly increasing the strength of the signal
Speed of Response: These small molecules diffuse quickly inside the cell, allowing for fast communication and rapid changes in cell activity.
Signal Distribution: A single second messenger can influence multiple pathways or activate various target proteins.
Regulation and Control: Second messengers allow for fine-tuning of responses based on concentration and feedback mechanisms.
Passing Through Membrane Barriers: Since many signaling molecules (like hormones) act at the membrane, second messengers are needed to carry the message into the cell where it can cause changes.
Explain the concept of membrane potential
the difference in electrical charge (voltage) across a cell’s membrane. It’s like a tiny battery:
The inside of the cell is more negative compared to the outside. This voltage difference is crucial for nerve impulses, muscle contractions, and cell signaling.
What establishes a resting membrane potential?
- Ion concentration differences (especially K⁺ and Na⁺).
- Selective ion permeability (especially K⁺ leaking out).
- The Na⁺/K⁺ pump that maintains these gradients.
This resting state is essential for cells to fire action potentials, respond to stimuli, and maintain homeostasis.
What ions are important for maintaining resting membrane potential?
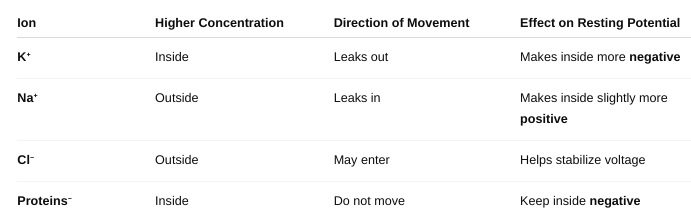
How are these ions distributed across the membrane, and what
maintains this
setup?
- Na⁺ = high outside, low inside
- K⁺ = high inside, low outside
- Cl⁻ = high outside, low inside
- Proteins⁻ = trapped inside
Maintained by:
- Na⁺/K⁺ pump (active transport)
- Selective ion permeability (especially K⁺ leakage)
- Trapped negative charges (proteins)
Together, these factors create and maintain the resting membrane potential, which is critical for nerve and muscle cell function.
What is the Nernst Equation and how is it used?
The Nernst equation is used to calculate the equilibrium potential (also called reversal potential) for a specific ion across a membrane.
This is the voltage at which there is no net movement of that ion in or out of the cell because the electrical and chemical (concentration) forces are balanced.
What Does It Tell Us?
- The equilibrium potential is the membrane voltage that would exactly balance the ion’s tendency to move down its concentration gradient.
- If the actual membrane potential ≠ equilibrium potential, the ion will try to move in the direction that brings it closer to equilibrium.
Describe the process contributing to the resting membrane potential of the cell.
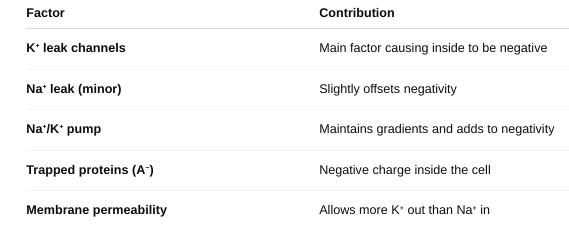
The resting membrane potential is the result of:
- Ionic gradients
- Selective permeability
- Active ion pumping
It sets the stage for action potentials, muscle contractions, and many cell functions.
Describe the different types of cell signaling.
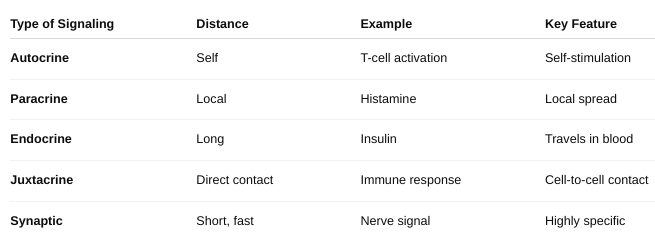
Distinguish among synaptic, endocrine, and paracrine regulation.
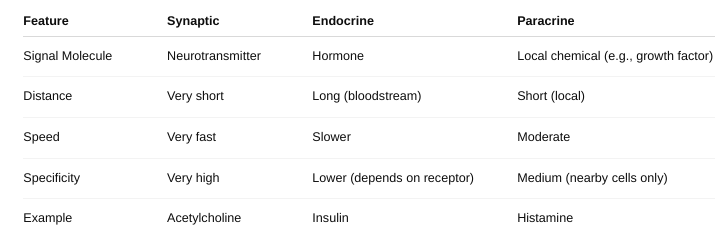
Identify the location of the receptor proteins for different regulatory molecules.
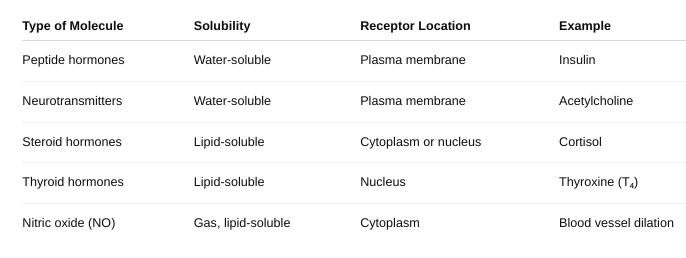
How do drugs work?
Drugs produce their effects by interacting with specific targets in the body—usually proteins such as receptors, enzymes, ion channels, or transporters. This interaction changes normal biological activity and causes a therapeutic or sometimes side effect.
- Absorption — How the drug enters the bloodstream.
- Distribution — How the drug spreads to tissues.
- Binding — Drug binds its target molecule (receptor, enzyme).
- Response — Drug-target interaction triggers a biological effect.
- Metabolism and Excretion — Body breaks down and removes the drug.
Drugs work by mimicking, blocking, or modifying normal biological processes.
Their effects depend on which target they interact with and how they interact (activate or inhibit).
Understanding drug action helps in designing safer, more effective therapies.
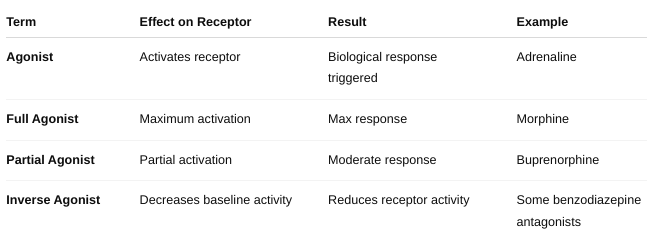
What is an antagonist?
An antagonist is a drug or molecule that binds to a receptor but does not activate it. Instead, it blocks or reduces the effect of an agonist (a molecule that normally activates the receptor).
Result: The antagonist prevents the receptor from producing its usual response.
Competitive vs non-competitive antagonist?

What is an agonist?
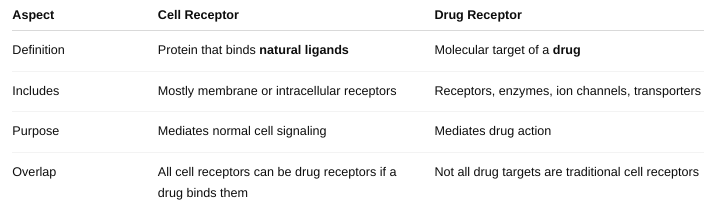
Define “drug receptor.” This may or may NOT be the same as a “cell
receptor.”
Differentiation between these two terms.
Can drugs produce “new cell functions?” Why or why not?
Generally, drugs do NOT create entirely new cell functions. Instead, they modify or influence existing cellular processes.
Drugs Work by Modulating Existing Targets
- Drugs bind to existing molecules in cells (receptors, enzymes, ion channels, transporters).
- They enhance, inhibit, or mimic normal physiological functions but do not create new cellular machinery.
Cells Have a Fixed Set of Functional Capabilities
- Cells are genetically programmed with a set of functions.
- Drugs influence how intensely or frequently these functions occur, not new functions.
No Creation of New Proteins or Structures by Drugs Directly
While some drugs can indirectly affect gene expression (like steroids influencing protein synthesis), this is a modulation of existing functions, not creation of entirely new ones.
What are the factors affecting drug-receptor interactions?
Affinity: How strongly a drug binds to its receptor. Higher affinity means the drug binds more tightly and is effective at lower concentrations.
Drug Concentration: More drug molecules increase the chance of receptor binding (up to receptor saturation).
Receptor Density: The number of receptors available on/in the cell affects the overall response.
Receptor Conformation (State): Receptors can have active or inactive shapes; drugs may bind preferentially to one state.
Competition: Other molecules (agonists, antagonists) competing for the same receptor affect drug binding.
Chemical Properties of the Drug: Size, shape, charge, hydrophobicity affect how well the drug fits and binds.
Environmental Factors: pH, temperature, and ionic strength can influence binding affinity.
Drug-receptor selectivity?
A drug’s ability to preferentially bind to a specific receptor subtype or a limited group of receptors rather than many different types.
Highly selective drugs tend to cause fewer side effects because they affect only certain pathways.
Selectivity depends on:
- Structural compatibility with the receptor binding site
- Differences in receptor subtypes (shape, amino acids in binding pocket)
- Drug concentration (higher doses may bind less selective sites)
What is affinity? (Figure 1-1 and Table 1-1)
Affinity influences the potency of a drug — drugs with higher affinity generally require lower concentrations to elicit a response. It affects how well a drug can compete with others (like natural ligands or other drugs) for receptor binding.
What factors define affinity?
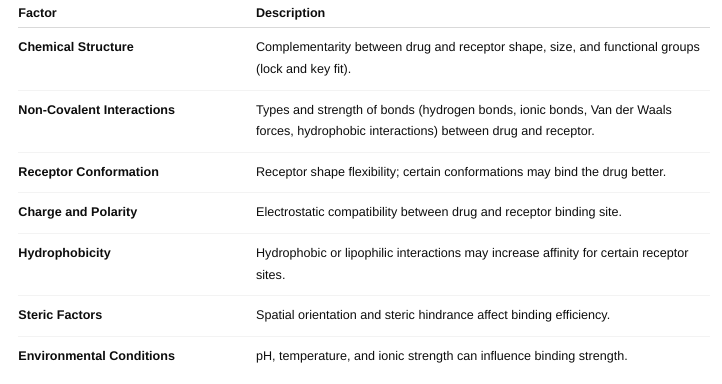
How does stereochemistry affect affinity? (Figure 1-2)

What is a racemic mixture?
a 50:50 mixture of two enantiomers of a chiral molecule.
What are the TWO cell-type drug-specificity strategies? (Cell-type specificity)

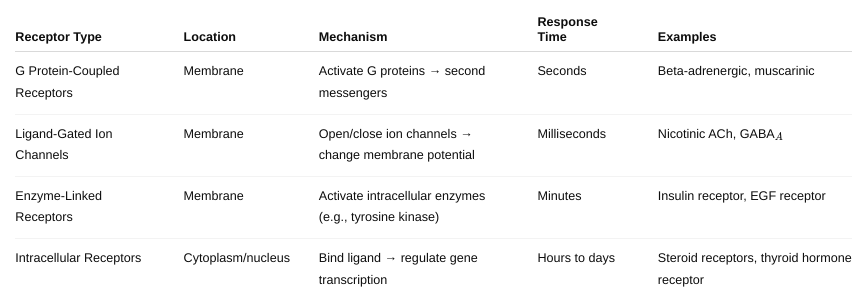
What are the major types of drug receptors and how do they work? Be
detailed.
Figure 1-3, 1-4, 1-5, 1-6, 1-7, 1-8, 1-9; Table 1-2, 1-3
What are the different mechanisms of tachyphylaxis? (Figure
1-10;
Table 1-6)
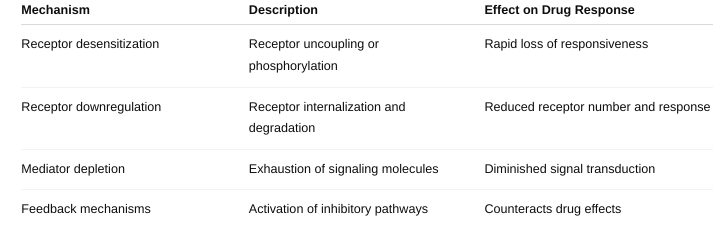
Tachyphylaxis is a rapid decrease in the response to a drug after repeated or continuous administration
Why is tachyphylaxis needed?
It’s a protective cellular mechanism to prevent overstimulation and damage from continuous exposure to certain drugs or signals.
Prevents excessive cellular responses that could be harmful.
Helps maintain homeostasis by adjusting receptor sensitivity based on ligand exposure.
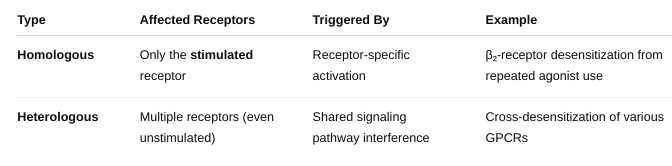
What is homologous vs heterologous?
Define: drug discovery
The process of identifying new candidate medications based on biological targets (like proteins, enzymes, or receptors) is involved in disease.
It includes:
- Target identification and validation
- Screening chemical compounds
- Finding molecules that might interact beneficially with the target
- Optimizing those molecules
➡️ Think of it as the very beginning of the journey to making a new drug.
Define hit
A "hit" is a chemical compound that shows promising activity against a drug target in an initial screen (often high-throughput screening).
Characteristics:
- Has measurable biological activity
- May bind or inhibit the target
- But may not be drug-like yet (it might be unstable, toxic, or weak)
➡️ A hit is like a rough gem — it shows promise but needs refinement.
Define lead
A lead compound is a hit that has been optimized for better effectiveness, safety, stability, and selectivity.
Characteristics:
- More potent and selective than a hit
- Shows desirable properties for becoming a drug
- Moves on to preclinical testing
➡️ A lead is a polished gem — a refined version of a hit that's ready for serious testing.
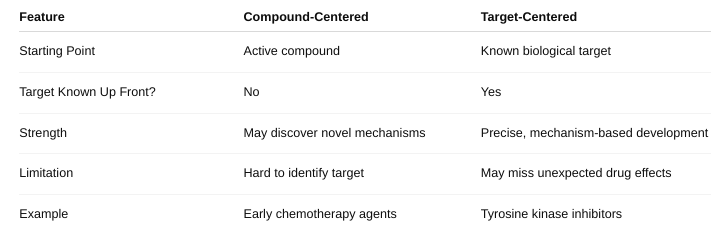
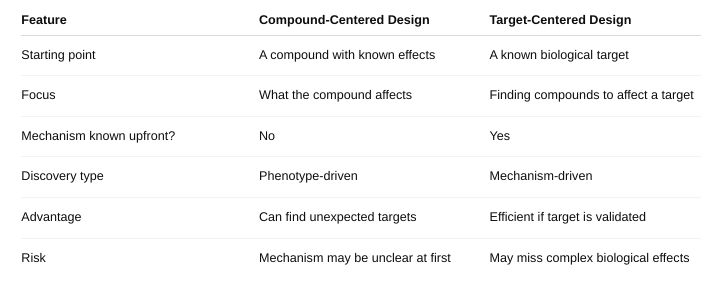
Define compound-centered and target-centered
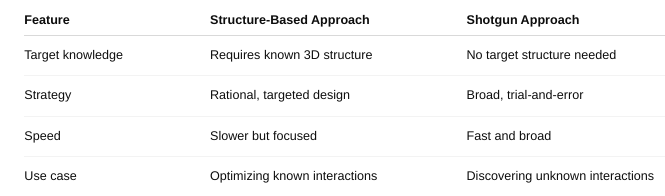
Define structure-based approach and shotgun approach
What is compound-centered drug design versus target-centered drug design?
How are analogues of natural compounds used in drug design, and what
are the
potential benefits?
modified versions of natural compounds (from plants, microbes, animals, etc.)
How they’re used:
Lead Optimization: A natural compound that shows some activity is used as a "lead compound."
Chemists then modify parts of the molecule to improve: Potency (how strongly it works), Selectivity (how specifically it acts on the target), Safety (reduce side effects), Solubility or bioavailability (how well it's absorbed in the body)
Overcoming Limitations: Natural compounds may degrade quickly, be hard to deliver, or have toxicity issues. Analogues are engineered to overcome these weaknesses.
Creating Patentable Drugs: Natural compounds themselves may not be patentable. Chemically altered analogues can be patented, making them commercially viable.
What are the potential benefits of analogues?

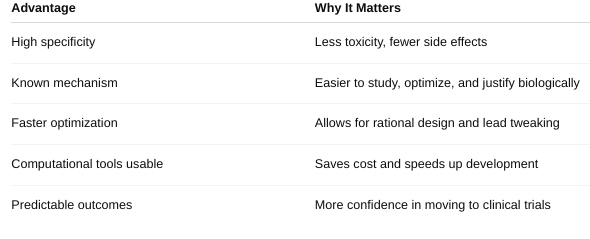
What are the advantages of target-centered drug design?
What is high-throughput screening?
a technology-driven process used in drug discovery to rapidly test thousands to millions of compounds for biological activity against a target (like a protein, enzyme, or cell). It’s like using a robotic assembly line to quickly find which chemicals might become drugs.
How It Works:
- Target is prepared (e.g., a protein involved in disease).
- A large library of compounds is stored in small wells on microplates (usually 96-, 384-, or 1536-well plates).
- Robots and automated machines: Add the compounds to each well, Mix them with the target, and Measure changes (e.g., enzyme activity, fluorescence, cell death).
- Software analyzes the results to find "hits" — compounds that show desirable activity.
How does high throughput screening facilitate drug discovery?
Speeds Up Discovery- Tests thousands of compounds in days, which would take years manually.
Identifies Hits Early- Rapidly finds "hit compounds" that affect the target — a crucial first step in drug development.
Works With Many Targets- Can be used on enzymes, receptors, ion channels, or even whole cells.
Unbiased Discovery- Useful even when the mechanism is not fully understood — lets the biology "speak for itself."
Data-Driven- Combines with machine learning and cheminformatics to spot patterns and optimize leads faster
How does combinatorial chemistry allow researchers to generate a large number of chemical compounds, and what is/are the advantages?
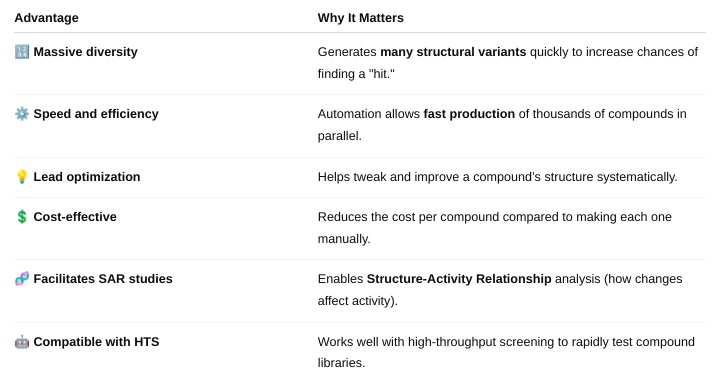
Combinatorial chemistry is a method used to rapidly create large libraries of different chemical compounds by systematically combining sets of building blocks in all possible ways.
What is structure-based drug design/rational drug design?
Structure-Based Drug Design (SBDD) — also called Rational Drug Design — is a method of designing drugs by using the 3D structure of a biological target (usually a protein or enzyme) to develop compounds that will bind to it in a specific and effective way.
Think of it like designing a key to perfectly fit a lock — where the lock is the target (e.g., an enzyme's active site) and the key is the drug molecule
How is it different from other methods of drug design?
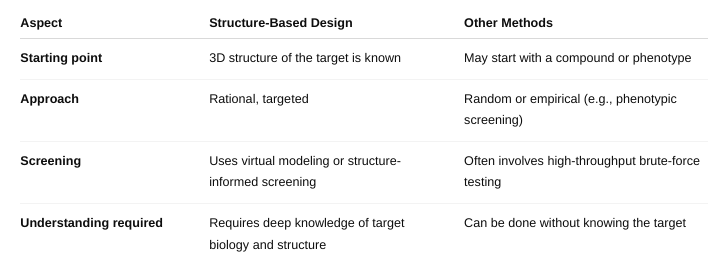
What are its advantages?

What is lead optimization? Where/when does it fit into the drug discovery timeline?
Lead optimization is a stage in the drug discovery process where scientists take a promising "lead compound" — a molecule that shows desired biological activity — and chemically modify it to improve its properties and make it a strong drug candidate.
1. Target Identification
↓
2. Hit Discovery (e.g.,
screening thousands of compounds)
↓
3. Hit-to-Lead (H2L)
→ Select promising hits as "leads"
↓
4. **Lead
Optimization**
↓
5. Preclinical Testing (in animals for
safety/toxicity)
↓
6. Clinical Trials (in humans)
↓
7. Regulatory Approval
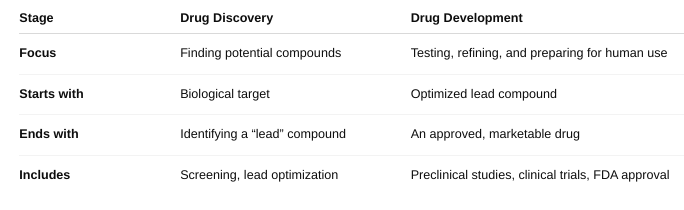
How does drug development relate to drug discovery? (Box 51-2)
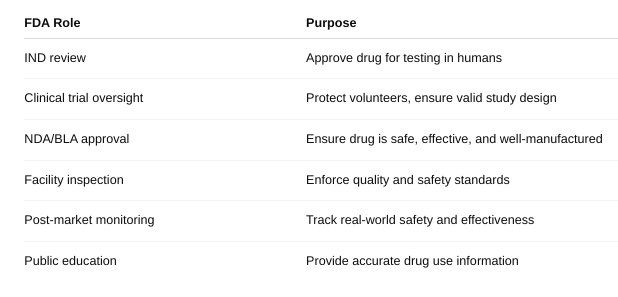
What role does the US Food and Drug Administration play in bringing drugs to market?
What is the estimated timeframe for drug development from drug discovery to approval of a new drug?
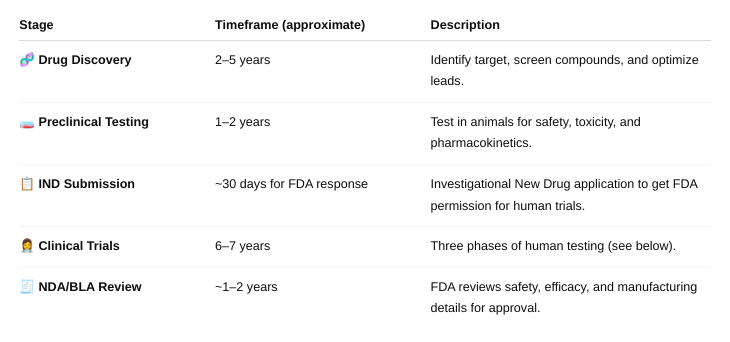
About 15 years
What percentage of drugs entering clinical trials achieves approval? (Figure 52-1)
Only about 12% of drugs that enter clinical trials (human testing) are eventually approved by the FDA.
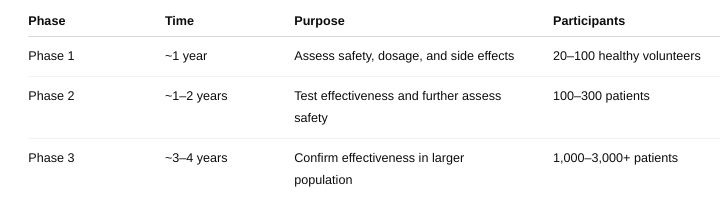
What are the three phases of a clinical trial?

What are the respective number of subjects involved, what is the length of each phase, and what is the purpose or focus of each phase?