BEGINNING OF CH. 11
BEGINNING OF CH. 11 (back of card)
Crystalline solids ________.
have ordered structures
________ liquid crystals are colored because the molecular layers are arranged in slightly twisted planes with respect to one another.
Cholesteric
What are the common types of smectic liquid-crystalline phases?
A and C
There are ________ types of smectic liquid-crystalline phases.
3
For a given substance that exhibits liquid-crystalline properties, the transition from solid to liquid-crystal state occurs ________.
at the melting point of the solid
In liquids, the attractive intermolecular forces are ________.
strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other
As a gaseous element condenses, the atoms become ________ and they have ________ attraction for one another.
closer together, more
Together, liquids and solids constitute ________ phases of matter.
the condensed
The strongest interparticle attractions exist between particles of a ________, and the weakest interparticle attractions exist between particles of a ________.
solid, gas
________ are particularly polarizable.
Large molecules, regardless of their polarity,
The ease with which the charge distribution in a molecule can be distorted by an external electrical field is called the ________.
polarizability
Elemental iodine (I2) is a solid at room temperature. What is the major attractive force that exists among different I2 molecules in the solid?
London dispersion forces
Hydrogen bonding is a special case of ________.
dipole-dipole attractions
________ is the energy required to expand the surface area of a liquid by a unit amount of area.
Surface tension
Which statements about viscosity are true?
(i) Viscosity increases as temperature decreases.
(ii)
Viscosity increases as molecular weight increases.
(iii)
Viscosity increases as intermolecular forces increase.
All of the above.
The shape of a liquid's meniscus is determined by ________.
the relative magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and its container
Viscosity is ________.
the resistance to flow
The property responsible for the "beading up" of water is ________.
surface tension
Heat of sublimation can be approximated by adding together ________ and ________.
heat of fusion, heat of vaporization
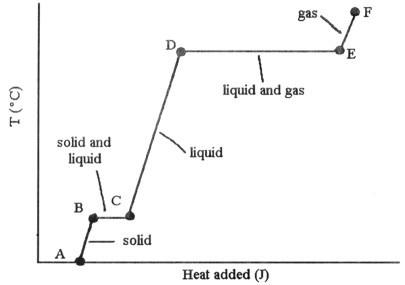
A-B solid
B-C solid and liquid
C-D liquid
D-E liquid
and gas
E-F gas
The ________ (is)are associated with the heat energy being used up to increase distances between molecules.
phase changes B → C and D → E
Large intermolecular forces in a substance are manifested by ________.
high heats of fusion and vaporization
high boiling point
low vapor pressure
high critical temperatures and pressures
Of the following, ________ is an exothermic process.
freezing
subliming
melting
boiling
freezing
A volatile liquid is one that ________.
readily evaporates
In general, the vapor pressure of a substance increases as ________ increases.
temperature
The vapor pressure of any substance at its normal boiling point is ________.
1 atm
Volatility and vapor pressure are ________.
directly proportional to one another
On a phase diagram, the critical pressure is ________.
the pressure required to liquefy a gas at its critical temperature
On a phase diagram, the critical temperature is ________.
the temperature above which a gas cannot be liquefied
On a phase diagram, the melting point is the same as ________.
the freezing point
BEGINNING OF CH. 12
BEGINNING OF CH. 12 (back of card)
A solid has a very high melting point, great hardness, and poor electrical conduction. This is a(n) ________ solid.
covalent network
Trends in melting points for metals can be explained with the ________.
electron-sea model
The ________ for Ge shows it to be a semiconductor, because the gap between the filled lower and empty higher energy bands is relatively small.
molecular-orbital model
All of the following are a type of solid except ________.
metallic
supercritical
ionic
covalent network
molecular
supercritical
Consider the following statements about crystalline solids:
(i) Molecules or atoms in molecular solids are held together via
ionic bonds.
(ii) Metallic solids have atoms in the points of
the crystal lattice.
(iii) Ionic solids have formula units in
the point of the crystal lattice.
(iv) Molecules in
covalent-network solids are connected via a network of covalent bonds.
Which of the statements is true?
(ii)
The ________ of light waves upon passing through a narrow slit is called diffraction.
scattering
What fraction of the volume of each corner atom is actually within the volume of a face-centered cubic unit cell?
1/8
What portion of the volume of each atom or ion on the face of a unit cell is actually within the unit cell?
1/2
________ have properties that depend on the manner in which the solid is formed.
Heterogeneous alloys
________ are examples of homogeneous alloys.
Intermetallic compounds
________ generally differ from compounds in that the atomic ratios of the constituent elements in the former are ________ and may vary over a wide range.
Alloys, not fixed
Of the following, ________ may be added to steel to modify its properties.
carbon and nickel
How many valence electrons do inorganic compounds contain if they are considered semiconductors?
4
All of the following are natural polymers except ________.
nylon
The empirical formula of an addition polymer ________.
is the same as that of the monomer from which it is formed
What happens to a polymer as it becomes more crystalline?
Its yield stress decreases.
Its density decreases.
Its
melting point decreases.
Its stiffness decreases.
None of the above is correct.
Natural rubber is too soft and chemically reactive for practical applications. ________ of natural rubber entails crosslinking reactive polymer chains with sulfur atoms.
Vulcanization
The formation of a ________ polymer generally involves the elimination of a small molecule.
condensation
All of the following are classified as a nanomaterial except ________.
buckminsterfullerene
carbon nanotubes
isoprene
graphene
All of the above are classified as nanomaterials.
The properties of graphene include ________.
high strength
large thermal conductivity
a zero energy gap
All of the above.
BEGINNING OF CH. 13
BEGINNING OF CH. 13 (back of card)
Hydration is a specific example of the phenomenon known generally as ________.
solvation
Pressure has an appreciable effect on the solubility of ________ in liquids.
gases
The phrase "like dissolves like" refers to the fact that ________.
polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
In a saturated solution of a salt in water, ________.
the rate of crystallization = the rate of dissolution
An unsaturated solution is one that ________.
has a concentration lower than the solubility limit
A solution with a concentration higher than the solubility allows is ________.
supersaturated
Molality is defined as the ________.
moles solute/kg solvent
Which one of the following concentration units varies with temperature?
Molality.
Mole fraction.
Mass percent.
Molarity.
Molarity.
The magnitudes of Kf and of Kb depend on the identity of the ________.
solvent
As the concentration of a solute in a solution increases, the freezing point of the solution ________ and the vapor pressure of the solution ________.
decreases, decreases
The ratio of the actual value of a colligative property to the value calculated, assuming the substance to be a nonelectrolyte, is referred to as ________.
the van't Hoff factor
Colligative properties of solutions include all of the following except ________.
an increase in the osmotic pressure of a solution upon the addition of more solute
the increase of reaction rates with increase in temperature
elevation of the boiling point of a solution upon addition of a solute to a solvent
depression of vapor pressure upon addition of a solute to a solvent
depression of the freezing point of a solution upon addition of a solute to a solvent
the increase of reaction rates with increase in temperature
The process of a substance sticking to the surface of another is called ________.
adsorption (not absorption)
All of the following are considered to be colloids except ________.
a foam
an emulsion
a homogeneous mixture
an
aerosol
All of the above are colloids.
a homogeneous mixture
Hydrophobic colloids ________.
can be stabilized by adsorption of ions
END
Good luck on the exam!
END (back of card)