Alkane
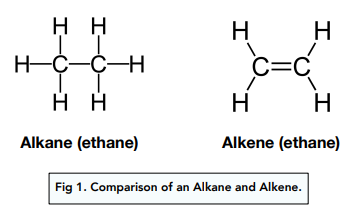
Alkene
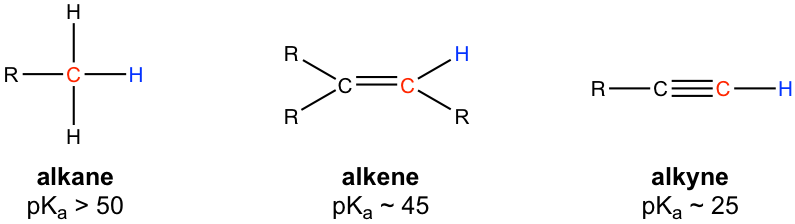
Alkyne
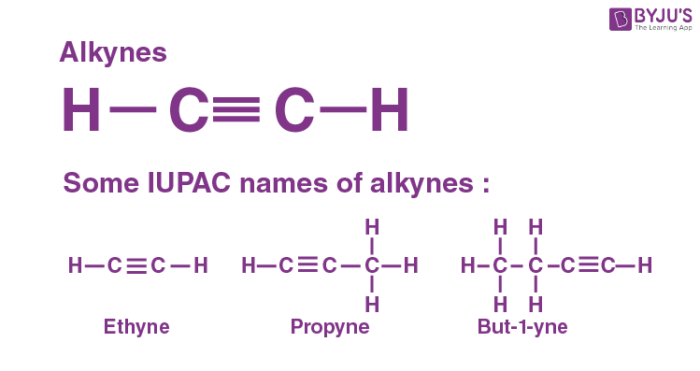
Is ethyne a hydrocarbon?
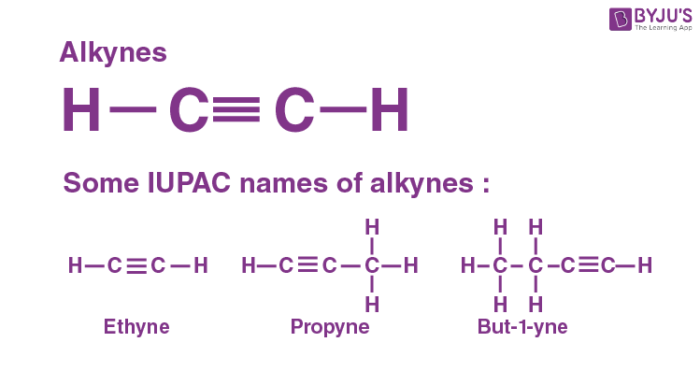
Yes, it is an unsaturated hydrocarbon (triple bond).
What is the common name of ethyne?
Acetylene
Are hydrocarbons soluble or insoluble in water?
Hydrocarbons are insoluble in water because they are nonpolar and water is polar.
Will hydrocarbon be in the top or bottom layer?
They will be the top layer because they are less dense than water.
Saturated hydrocarbon
- Molecules contain only single bonds.
- Alkanes.
Unsaturated hydrocarbon
- Have double or triple bonds.
- Alkenes and alkynes.
Write down the balanced equation for calcium carbide (CaC2) reacting with water to give off ethyne when reacted with water.
CaC2 + 2H2O -> Ca(OH)2 + C2H2
How many grams of ethyne (molar mass = 26.0 g/mol) would be given off if the limiting reagent in the reaction is calcium carbide (molar mass = 64.1 g/mol) and you start with 2.00 grams of it?
The equation of the reaction is;
CaC2(s) + 2H2O (l) ------> Ca(OH)2(aq) + C2H2(g)
We have been told that CaC2 is the limiting reactant.
Number of moles of CaC2 = 2g/ 64 g/mol = 0.03 mol
If 1 mole of CaC2 yields 1 mole of acetylene
0.03 moles of CaC2 yields 0.03 moles of acetylene
Mass of acetylene = number of moles * molar mass
Molar mass of acetylene = 26 g/mol
Mass of acetylene = 0.03 moles * 26 g/mol
Mass of acetylene = 0.78 g of acetylene.
Aromatic hydrocarbon
Cyclic, planar compounds that resemble benzene in electronic configuration and behavior.
By which test can you identify if a compound is saturated or unsaturated?
Bromine test
What is a drying agent?
- A chemical used to remove water from an organic compound that is in solution.
- Calcium chloride (CaCl2), Calcium oxide (CaO)
A hydrocarbon is saturated if ___ are present.
___ are saturated hydrocarbons.
A hydrocarbon is unsaturated if ___ are present.
___ are unsaturated hydrocarbons.
- only single bonds
- Alkanes
- any multiple bonds
- Alkenes
In general, when a hydrocarbon is added to water, the hydrocarbon will ___ the water because hydrocarbons are ___ than water.
- float above
- less dense
In general, when hydrocarbons like oil are added to water, the two liquids ___ because hydrocarbons are ___ and water is ___.
- do not mix
- non-polar
- polar
The bromine test shows the presence of ___.
A positive bromine test appears as ___.
A negative bromine test appears as ___.
- alkenes
- a colorless solution
- an orange solution
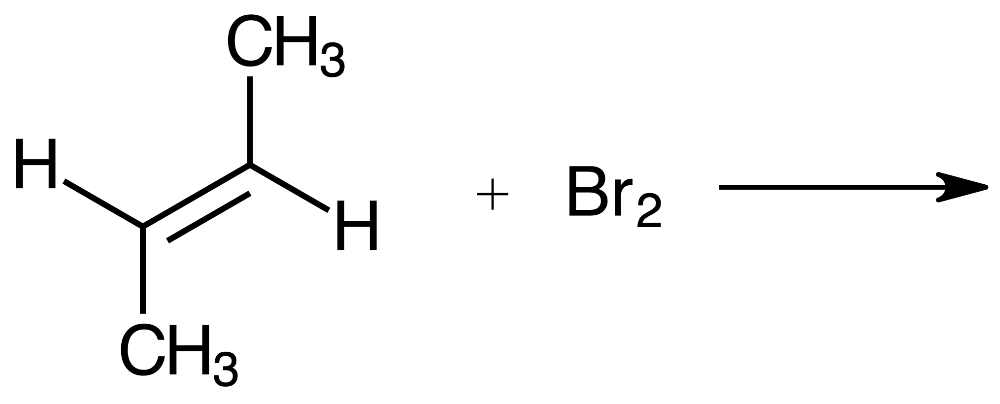
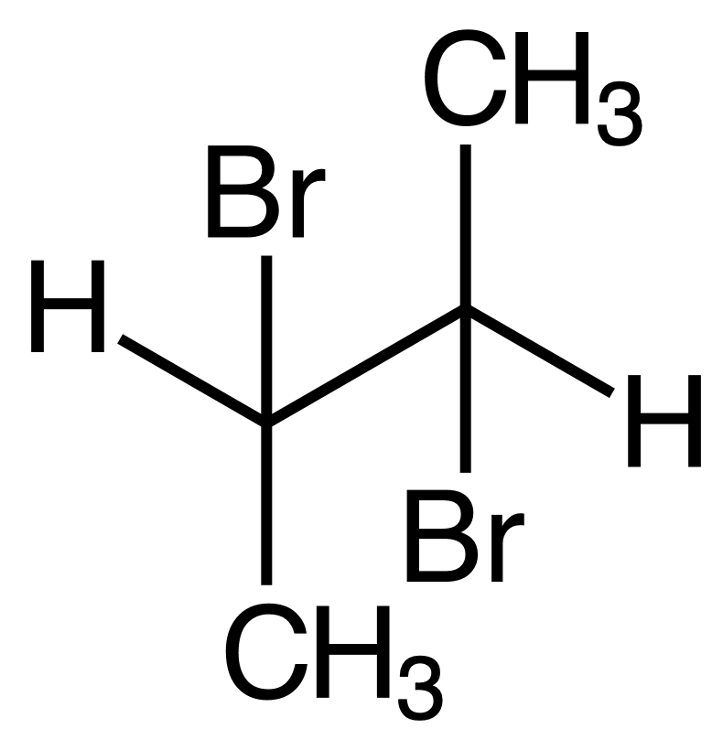
Consider the reaction of bromine with the pictured alkene. Which structure shows the correct bonding for the product?
Baeyer's test, also known as the permanganate test, shows the presence of ___.
A positive Baeyer's test appears as ___.
A negative Baeyer's test appears as ___.
- alkenes
- a brown precipitate
- a purple solution
The biggest concern of working with hydrocarbons in lab is that many of them are ___.
Therefore, hydrocarbons should be handled ___ to contain any associated vapors with that hazard.
- flammable
- in the hood
What is a nucleophile?
Substances that tend to donate electron pairs to electrophiles in order to form chemical bonds with them.
What is an electrophile?
A molecule that is electron deficient and can accept a pair of electrons to make a covalent bond.
What do you mean by "back-side" attack in substitution reaction?
- The nucleophile attacks the electrophilic center on the side that is opposite to the leaving group. When a backside occurs, the stereochemistry of the product doesn't stay the same.
- SN2 reaction
Protic solvent
- Protic solvents have an O-H or N-H bonds and can hydrogen-bond with themselves.
- Water (H2O), Ammonia (NH3), Ethanol (C2H6O)
Aprotic solvent
- Solvents in which no hydrogen bonding takes place or they neither donate nor accept the proton (hydrogen).
- Ex: Acetone (C3H6O), Dichloromethane (CH2Cl2)
SN1 reaction
- A nucleophilic substitution reaction where the rate-determining step is unimolecular.
- Commonly occurs with carbonyl compounds as well as benzene.
- Solely dependent on substrate concentration, as carbocation formation has a high activation energy barrier, and overcoming this barrier takes time.
SN2 reaction
- Reaction occurs only when the occupied lone pair orbital of the nucleophile donates electrons to the unfilled antibonding orbital between the carbon center and leaving group.
- Depends on substrate concentration and nucleophile concentration.
- Commonly occurs with alkyl halides and also benzoins.
Solvent for SN1 reaction
- Polar protic
- H2O, ROH
Solvent for SN2 reaction
- Polar aprotic
- Acetone
What is a carbocation?
A molecule in which a carbon atom has a positive charge and three bonds.
How many different types of carbocations are possible?
- Three different types: primary, secondary, tertiary.
Stability of carbocation
tertiary > secondary > primary
Which one best describes the mechanism of Nerolin synthesis?
SN2
A primary alkyl halide would prefer to undergo ___.
SN2
A tertiary alkyl halide would prefer to undergo ___.
SN1
Know which reagent has been used as a nucleophile, electrophile, and base in Nerolin synthesis.
- Nucleophile: 2-naphtoxide
- Electrophile: iodoethane?
- Base: potassium hydroxide
What is the color of bromothymol blue indicator in acidic and basic condition?
- Acidic: yellow
- Basic: blue
SN1 reaction rate depends on
Substrate only (unimolecular)
SN2 reaction rate depends on
Substrate and nucleophile (bimolecular)
SN1 Energy barrier
Carbocation stability
SN2 Energy barrier
Steric hindrance
SN1 Alkyl halide structure (electrophile)
3° > 2° > 1°
SN2 Alkyl halide structure (electrophile)
1° > 2° > 3°
SN1 Nucleophile
Weak (generally neutral)
SN2 Nucleophile
Strong (generally negatively charged)
SN1 Solvent
Polar protic (ex: water, alcohols which stabilize the resulting (charged) carbocation that results from loss of the leaving group. These also tend to be the nucleophiles for these reactions as well).
SN2 Solvent
Polar aprotic (ex: DMSO, acetone, acetonitrile, or DMG that are polar enough to dissolve the substrate and nucleophile but do not participate in hydrogen bonding with the nucleophile).
SN1 Stereochemistry
Mix of retention and inversion
SN2 Stereochemistry
Inversion only
When it is time to end a reflux, first ___ and then turn off the heat.
___ until the system has cooled.
- lower the heat source
- Leave the condenser water on
Many reflux procedures involve a required length of time for the reflux to occur. When should you start timing the reflux for the procedure?
When the reflux ring stabilizes the condenser
If crystal growth does not start on its own after the solution in the flask returns to room temperature, identify the best ways to promote this process.
- Scratch the bottom of flask gently with a stirring rod.
- Add a bit of solid as a seed crystal.
When working in the fume hood, it is important to make an effort to minimize ___.
Only keep items in the hood if they are being used for ___. Do not ___ chemicals in the fume hood unless instructed to do so.
- clutter
- the current experiment
- store
When working in a fume hood, what is the best position of the hood sash?
As low as possible, no more than halfway up.
An SN2 reaction is a type of ___ in which the nucleophile attacks the electrophile ___ a leaving group leaves. The rate of the reaction depends on the concentration of ___.
- substitution reaction
- at the same time
- both reactants
Consider the SN2 reaction of an alcohol with an alkyl halide in the presence of base.
The ___ should be added to the alcohol first to ___ the alcohol and allow it to attack the ___.
- base
- deprotonate
- alkyl halide
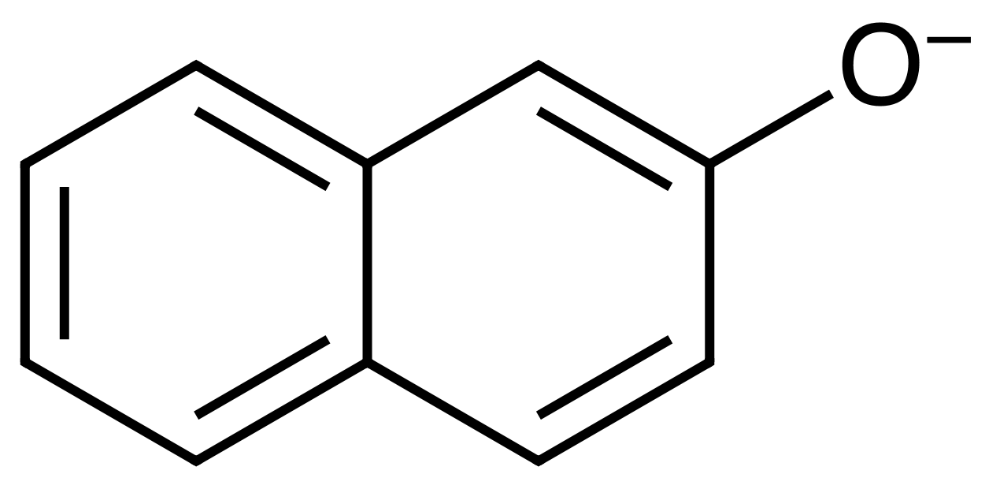
Nucleophile
KOH
Base
I-
Leaving group
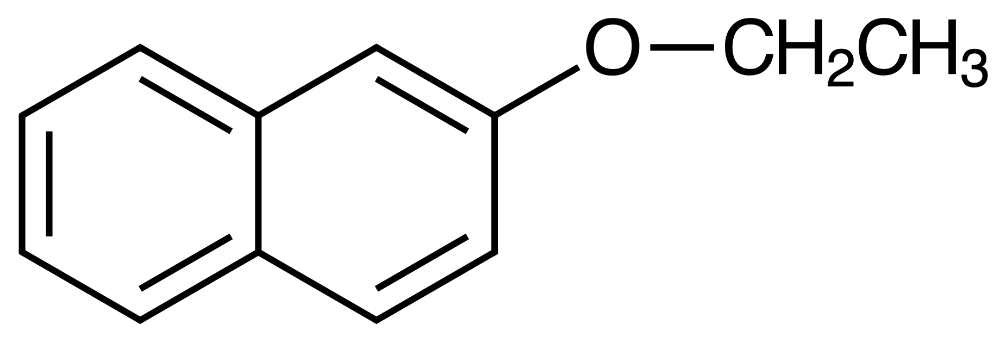
Product
I-CH2CH3
Electrophile
Why is potassium hydroxide preferred over sodium hydroxide in organic reactions?
Potassium hydroxide is more soluble in organic alcohols.
The term SN1 describes a reaction that is a ___ involving ___.
- nucleophilic substitution
- one molecule in the rate-determining step
The reaction rate is constant regardless of the amount of reactant in solution.
Zero order
An increase in the concentration of the reactant in solution causes the reaction rate to increase exponentially.
Second order
The reaction rate increases in direct propotion to the concentration of the reactant in solution.
First order
Bromothymol blue is an indicator that appears ___ in basic conditions and ___ yellow in acidic conditions.
- blue
- yellow
First order reaction
ln[reactant] vs time
Zero order reaction
[reactant] vs time
Second order reaction
1/[reactant] vs time
Suppose you are performing a titration. At the beginning of the titration, you read the titrant volume as 2.59 mL. After running the titration and reaching the endpoint, you read the titrant volume as 26.97 mL.
What volume, in mL, of titrant was required for the titration?
24.38
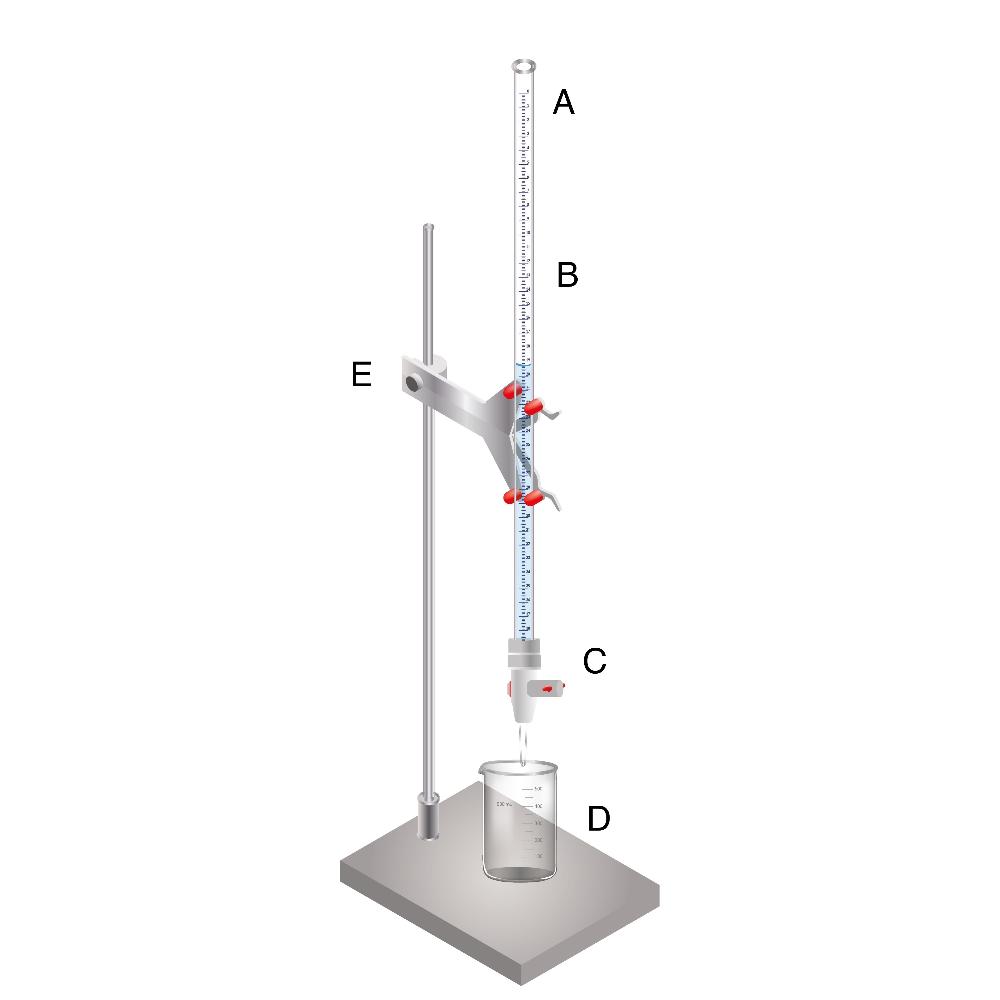
A = 0.00 mark
B = barrel
C = stopcock
D = collection flask
E = buret clamp
Which changes can be made to a chemical reaction in order to increase the rate constant?
- Increase the temperature
- Add a catalyst
What is the slowest step of an E1 reaction?
How does this relate to an SN1 reaction?
- Carbocation formation
- The energy barrier for SN1 reaction is carbocation stability.
Why can more than one product sometimes be formed during an E1 reaction?
Because of the formation of different carbocation intermediates and different reaction pathways. The stability of the carbocation intermediate and steric hindrance can influence the major and minor products.
Which functional group shows a positive bromine test? What will be the color change for the reaction?
- Alkene, aldehyde
- Yellow to neutral
What will be the color change if you test unsaturation with KMnO4?
Purple to brown
Know the mechanism for an acid-catalyzed dehydration of alcohol.
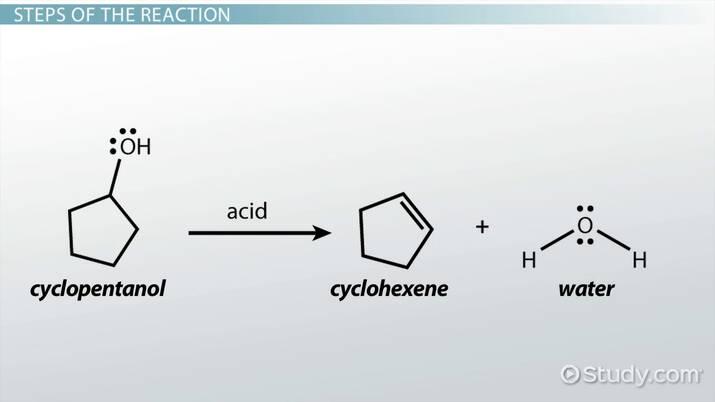
Describe the complete role of the acid catalyst in the dehydration of an alcohol.
The acid protonates the hydroxyl group and then the conjugate base deprotonates an adjacent carbon.
What is the end product of dehydration of cyclohexanol.
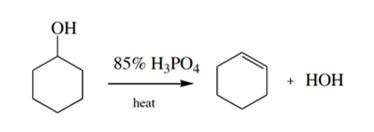
Alkene cyclohexene.
Which of the following best describes the mechanism of dehydration of an alcohol?
E1
Be able to identify the parts of the reflux set-up.
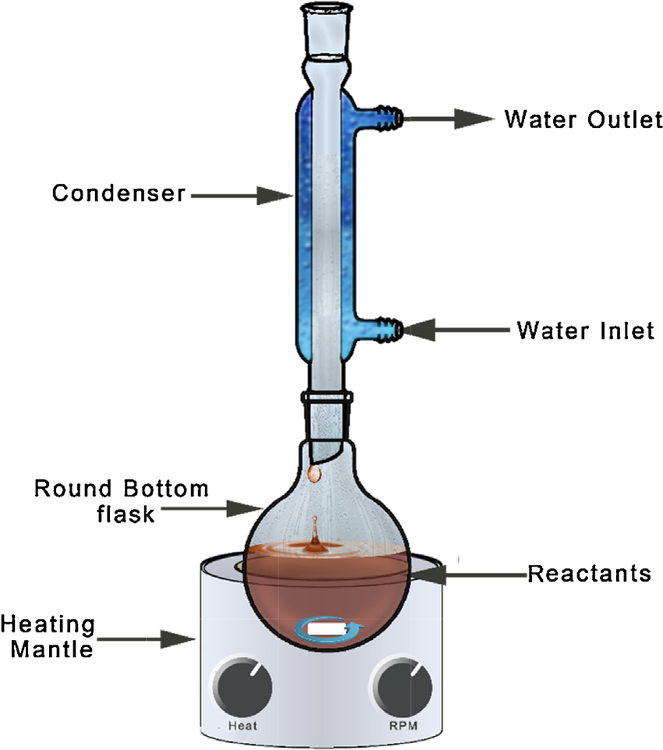
What was the dehydrating agent you have used in dehydration of cyclohexanol reaction?
Phosphoric acid????
Which statement is not true of nucleophilic substitution reactions?
Rates of both SN1 and SN2 increase with higher concentration of the nucleophile.
Which one of the following alkyl halides will undergo SN1 most readily?
(CH3)3C-I
Which of the following statements is not correct about SN2 reaction of alkyl halides?
SN2 mechanism is predominant in tertiary alkyl halide
Which of the following cannot react as a nucleophile?
(CH3)4N+
The dehydration of a secondary alcohol, like cyclohexanol, is a mechanism that occurs in ___.
First, the alcohol is protonated to ___, creating ___ intermediate.
Then, a ___ is removed, moving the electrons from that bond to make a ___ bond.
- two steps
- leave as a water molecule
- a cation
- hydrogen ion
- carbon-carbon double
How can a catalyst be recognized in a mechanism?
The catalyst is used and then regenerated in a later step.
In the presence of acid and heat, ___ will undergo a more effective dehydration reaction because it is a ___ alcohol and the ___ in the intermediate of the mechanism will be more stable.
- cyclohexanol
- secondary
- cation
If the dehydration of an alcohol is successful, what changes would be seen in the IR spectrum for the product compared to the starting material?
- The disappearance of an O-H band from the starting material
- The addition of a C-C double bond band in the product
- The disappearance of a C-O band from the starting material
Why does the dehydration of an alcohol more often use concentrated sulfuric acid, H2SO4, as the acid catalyst rather than dilute hydrochloric acid, HCl?
- The additional water solvent from a dilute solution could reverse the dehydration reaction
- The presence of the chloride ion could result in a competing substitution reaction
When performing the acid-catalyzed dehydration of an alcohol, the yield of product can be increased by ___ during the reaction.
Dehydration is ___, and removing the product from ___ reduces the ___ reaction.
- distilling off the product
- reversible
- the reaction flask
- hydration
What are the concerns presented by overheating a distillation to a dry flask?
- The empty glassware might heat quickly, igniting vapors from the distillation
- The remaining solid residue might contain explosive peroxides
Where should the tip of the thermometer be placed in a microscale distillation set-up?
At or slightly below the side arm of the distillation head.