Spectroscopy
the theoretical approach to the science of studying the interaction between matter and radiated energy
Spectrometry
the practical application of spectroscopy
Spectrometry uses instruments called
spectrometers
Spectrophotometry
the method used to measure how much achemical substance absorbs light as a beam of light passes through asample solution
Spectrophotometers
the instruments used to quantitatively measure the reflection or transmission properties of a material as a function of wavelength (a spectrum)
Spectrum
the reflection or transmission properties of a material as a function of wavelength
Light behaves as: (2)
a WAVE and a PARTICLE
Light as a WAVE
- has a wavelength and frequency
- exhibits the wave phenomena of interference, diffraction, and reflection.
- Wave properties govern light behavior such as interference and diffraction
Wave properties govern light behavior such as
interference and diffraction
Light as a PARTICLE (a photon)
- carries a discrete energy that can be absorbed or emitted by a molecule.
- The interaction of light with chemicals is described using the particle nature of light—the photon and its energy
Light waves consist of
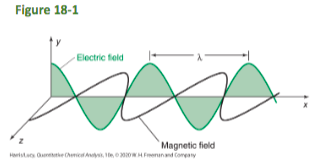
perpendicular, oscillating electric and magnetic fields
Wavelength, λ
the distance between wave crests
Wavelength units
m, µm, nm
Wavenumber (v)
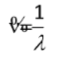
a measure of spatial frequency
(v = 1/λ)
Frequency, ν
- the number of oscillations per second of the wave
- Frequency has
units of hertz (s-1). - 1 Hz = 1 oscillation per second
Speed of light, c, formula
- The product of wavelength times frequency
- νλ = c
- In a vacuum, all light travels at the same speed.
c = 2.998 × 108 m/s
Light has a duality of
Wave-Particle
What is the smallest amount of light that can be generated by a light source?
A photon is the smallest amount of light that can be generated by a
light source.
A photon is a particle of light.
The energy of a photon can be calculated from its
frequency
Photon energy formula
Ephoton = hν
where
h = Planck’s constant = 6.626 × 10-34 J∙s
ν = the
frequency of the photon in units of hertz, s-1
E =
the energy of the photon in units of joules, J
Constants
c = 2.998 × 108 m/s (the speed of light in a
vacuum)
h = 6.626 × 10-34 J∙s (Planck’s constant)
Equations
v = 1/λ (conversion equation between wavelength and wavenumbers)
λν = c (equation for speed of light in a vacuum)
Ephoton = hν (equation for energy of a photon of light)
E = hν = hc/λ = hcv
Our eyes only see a fraction of the light in the universe. T/F?
True
Spectrophotometry
Any technique that uses light to measure chemical concentrations
Absorption Spectrophotometry
Any technique that uses the absorption of light to measure chemical
concentrations