How is research performed?
1) Formulate a question
2) Research (see what's been done before)
3) Selecting analytical procedure:
- use gold standard method: a tried and true practice that you will use. good validity.
4) sampling
- composition sampling--> map out the area
- matrix: anything in your sample that is not your analyte
- sampling is really important!!
- Ex: like making blood last longer via a dilution. Super sensitive to changes in concentration.
5) sample preparation - get the analyze of interest separated
- Separation from the matrix (can take a long time)
Chemical Analysis
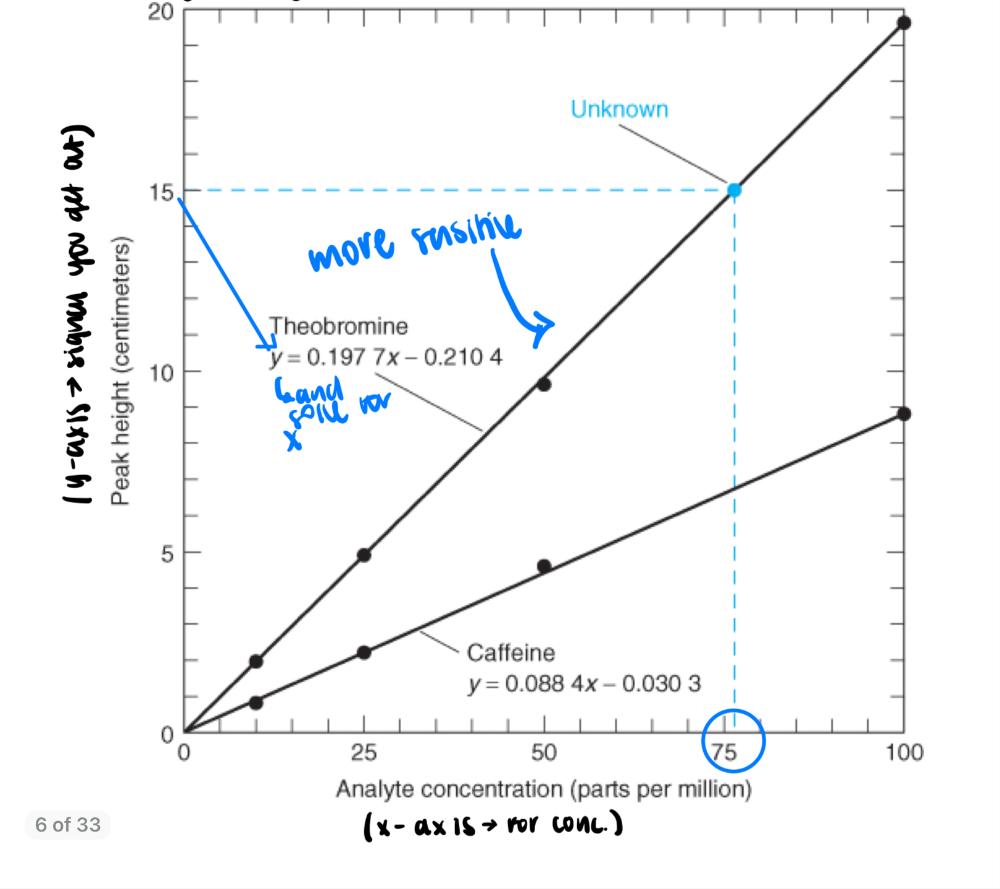
- make a calibration plot
- use standards (look for straight lines) --> make your own standard of known concentration
- calibration: creating standard of known concentrations and analyzing them in the same way as your analyte.
- instrumentation is really important especially with troubleshooting.
- replicate analysis: run at least 3 --> because cost effecting to do only 3
Ex:
Molarity
moles solute / L solution
Molality
moles solute/ kg solvent
ppm units
In terms of volume: mg/L or μ/mL. If speaking in terms of mass, μg/g.
pub unites
In terms of volume: μg/L. In terms of mass, ng/g
How to calibrate glassware?
note: always introducing error
to calibrate: use water because density (temp. dependent)
--> mass out how much water it takes to calibrate out a flask. Then do this 3 times. Weigh it out .
always use a volumetric flask
Pipets
1) transfer pipet, only measures single sample. More accurate less error the line is already calibrated.
2) Measuring pipet, want values that aren't always set.
3) Micropipet, for really small volumes. Stable aqueous solutions. Many organic solvents (not chloroform(. Not resistant to concentrated HNO3 or H2SO4.
Repition
try to do at least 3 replicates. Tell us about precision. Try your method and see how accurate your results are to the gold standard method.
How do you know if your technique is reliable? Accurate?
Accuracy: how close our value is to the true value. Test this by using a secondary technique "the gold standard". Test this by making external standards
Absolute error = |Xi - Xt|
Relative error = |Xi - Xt| / Xt x 100% . Want relative error less than 10%
Experimental Error
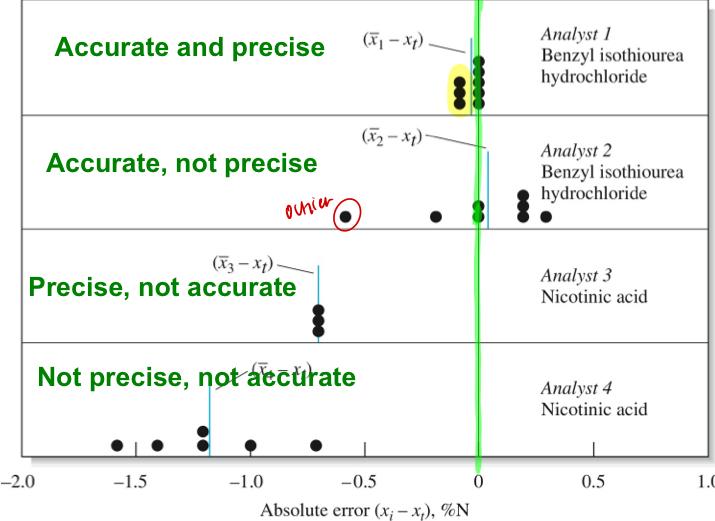
Types of Errors
- Random error: arrises from uncontrolled and uncontrollable variables in your measurement. Equal chance of being positive or negative.
--> effects greatly precision
--> Ex: reading a buret/scale indifferent noise, electrical fluctuations in your instrument, vibrations of drafts, environmental effects based on location or proximity or temp.
- Systematic error: affects accuracy. Arises rom flaws in your equipment in experimental design. Reproducible error. Constant (same absolute error but relative error changes with sample size.
--> proportional error (absolute error changes with sample size but relative error is constant).
--> How to check for accuracy?
1 . use a different analytical method
2. use a certified standard
3. run a blank (-) control, or run (+) control
How to reduce random error?
- physical thing to protect your sample (like casing around balance)
- more replicates (increase sample size)
- average data
Sig Figs for Logs
The number of figures in the mantissa (number of digits after decimal point in logarithm) should equal the number of significant figures in the quantity.
Propagation of Uncertainties for Random Errors: Addition or Subtraction

Propagation of Uncertainties for Random Errors: Multiplication or Division
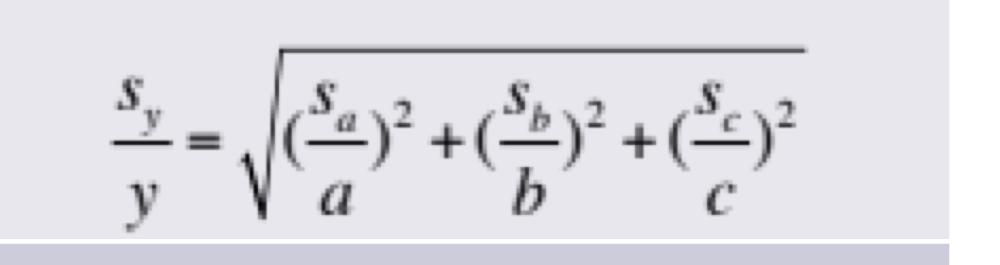
Propagation of Uncertainties for Random Errors: Exponentiation
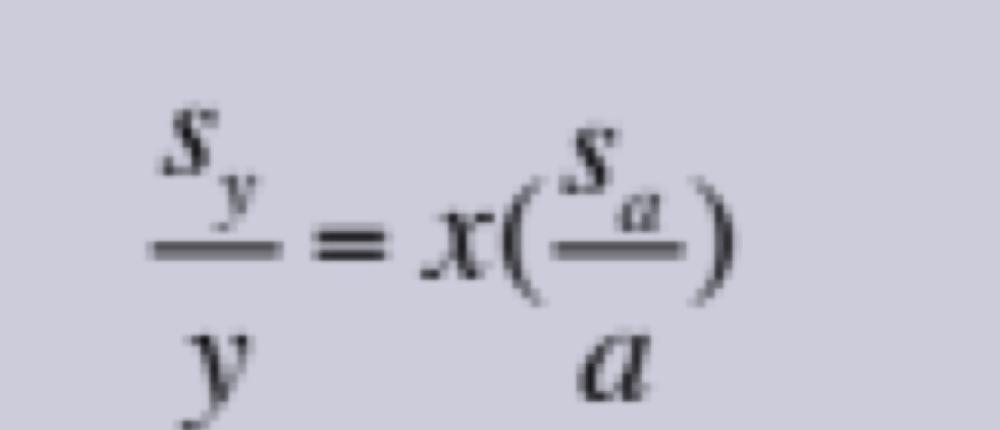
Propagation of Uncertainties for Random Errors: Logarithm
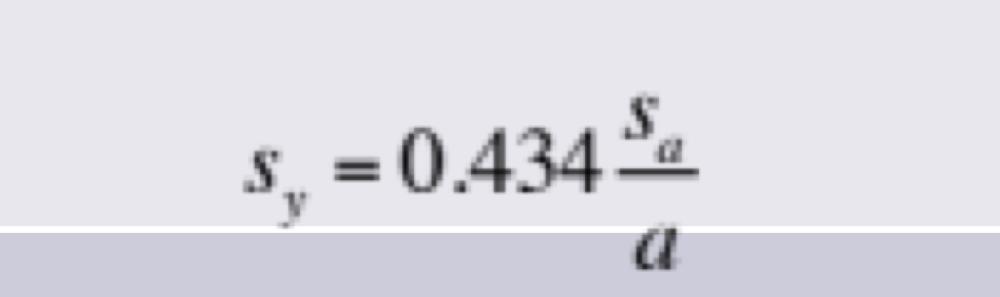
to find pH
Propagation of Uncertainties for Random Errors: Antilogarithm
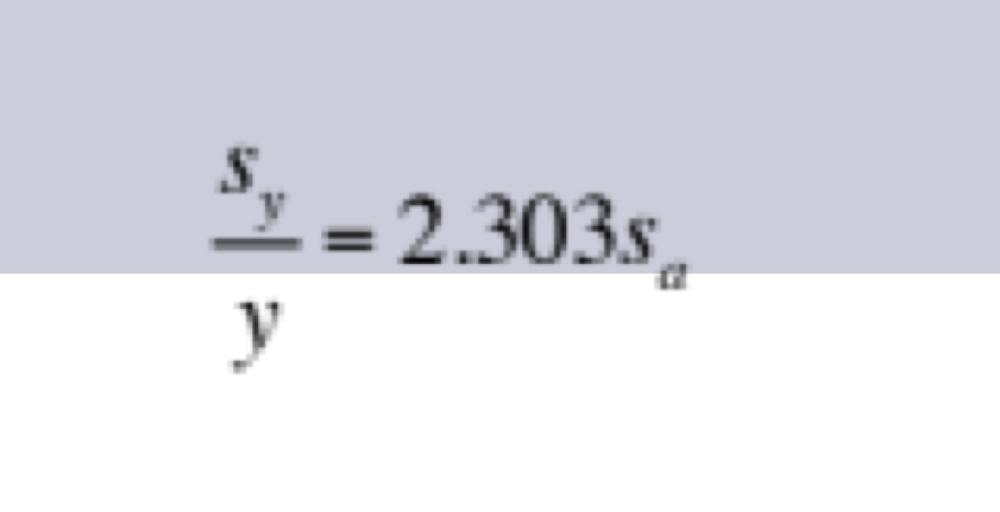
to find H+
If you have an uncalibrated pipet what happens to standard deviations when propagating error
Random error now becomes systematic error. Basically, take how many n multiples you have to equal the (final) measured out volume and multiply that by the (constant) standard deviation.
Gaussian Distribution
the broader the distribution the greater s is and less precision. i.e. you want a steep narrow curve
z score
how many standard deviations you are from the mean
standard deviation of the mean
σn is a measure of the uncertainty of the mean of n measurements
σn = σ / n1/2
larger samples = smaller SEM
makers error look smaller
F test
to decide whether 2 S.D. are SIG DIFF from each other
--> If F calc > Ftable then data sets are statistically different and precision is not the same
Confidence intervals
Use to compare mean measure by different methods. Allows us to state a range of values that include the population mean. Confidence interval is calculated to determine if the known value is significantly different from the measured value.
T-test type 1
compare an experimental mean with an accepted mean. think given means then use CI equations. Also deals with accuracy