What are the steps involved in Vitamin D synthesis?
7-cholecalciferol is present in the skin, upon exposure to ultraviolet light, it is transformed into the inactive form of Vitamin D. This inactive form, must first go to the liver and be 25-hydroxylated, and become 25 hydroxy Vitamin D. This then goes to the kidneys to be 1-hydroxylated to becomev1,25-dihydroxy Vitamin D (The active form of Vitamin D which will have the actions on the gut, bone, and kidneys as described above.
What are the effects at the Kidneys for vitamin D, parathyroid hormone, calcitonin
Vitamin D: ↑ Ca reabsorption (↓ Ca excretion)
↑
PO4 reabsorption (↓ PO4 excretion)
Parathyroid Hormone:
↑ Ca reabsorption (↓ Ca excretion)
↓ PO4 reabsorption
(↑ PO4 excretion)
- Calcitonin:
↓ Ca reabsorption (↑ Ca excretion)
What are the effects on the GI Tract for vitamin D, parathyroid hormone, calcitonin
Vitamin D: ↑ Ca absorption
Parathyroid Hormone:↑ Ca absorption
Calcitonin: -
What are the effects on the Bones for vitamin D, parathyroid hormone, calcitonin
Vitamin D: ↑ bone mineralization(Ca deposition into bone)
Parathyroid: Hormone:↑ bone resorption(harvesting Ca and PO4)
Calcitonin: Inhibited osteoclastic activity (Inhibited bone resorption)
What is the primary difference between Vitamin D and PTH in bone Calcium metabolism?
Vitamin D stores Ca in the bones and PTH makes free Ca2+ out of the osteoid matrix
What are the two ways that we can decrease free Calcium?
Excrete Ca in the kidneys or increase Phosphate
How does the following substance cause hypercalcemia: Hydrochlorothiazide (HCTZ)
Reduction in renal Calcium excretion
How does the following substance cause hypercalcemia: Lithium
Increases the body’s “set point” for Calcium
How does the following substance cause hypercalcemia: Vitamin A
excess Activation of osteoclasts (bone resorption)
How does the following substance cause hypercalcemia: Vitamin D
excess Increased absorption of Ca in the GI tract and decreased Ca excretion in the kidney
How does the following substance cause hypercalcemia: Oral calcium excess (milk alkali syndrome)
Hypercalcemia, alkalosis, peptic ulcer disease, and renal impairment
What may tell you that a person with a cough and nodule in his lungs on a chest X-ray, may have Squamous Cell Lung Carcinoma?
Elevated PTHrP levels or hypercalcemia
A 37-year-old male who smokes 1.5 packs per day comes to the
physician’s office to get checked out. He has had a productive cough
for the past few months, but it hasn’t bothered him much. The most
troublesome fact for him is that he is a construction worker and he
has been feeling so tired lately. He has not been able to function.
The physician orders a Chest X-Ray in the office and there is a nodule
in the upper left lobe of the lung. He wants to order some tests to
check the serum Calcium. What tests would you recommend to assess for
paraneoplastic hypercalcemia (Cancer causing the
hypercalcemia)?
A. Serum Calcium
B. PTHrP
C. N-terminal
PTH
D. C-terminal PTH
E. Intact PTH
The correct answer is B) PTHrP. Remember this is a protein that
functions a whole lot like PTH, but it’s not. It’s production is also
unregulated by the body. This will lead to an unregulated increase in
Calcium levels. PTHrP is commonly associated with squamous
cell
carcinoma of the lung, melanoma, and breast cancer.
2. A 57-year-old female presents to the clinic with the recent
history of kidney stones, despite being adequately hydrated. She just
wanted to make sure that everything was okay. She asks about the
possibility of her getting too much Vitamin D because of the frequent
boat trips that she has been taking and sunbathing. The physician
astutely responds with the statement that he’s not too worried that
the kidney stones are caused by excess Vitamin D. Why is that?
A.
Vitamin D stores Calcium in the bones
B. Vitamin D increases GI
absorption of Ca
C. Vitamin D increases the reabsorption of Ca in
the kidneys
D. Vitamin D increases the reabsorption of PO4 in the kidneys
The correct answer is C) Vitamin D increases the reabsorption of Ca in the kidneys. All of these answers correctly describe actions of Vitamin D, but they’re NOT the primary reason why the physician is not worried about Vitamin D producing kidney stones. Think, the reason is because Vitamin D increases the reabsorption of Ca in the kidneys will cause less Ca to be in the tubular fluid, therefore the more likely reason for her would be either dehydration (which would increase the relative concentration of Ca in the kidneys), or a problem related to PTH.
A 53-year-old male presents to his primary care physician with
longstanding hypertension (138/102). He is taking blood pressure
medication including an ACE inhibitor, HCTZ, Metoprolol, and a statin
for his elevated lipid levels. The physician notices that he is a
little more depressed than usual. He admits to constipation over the
past week, more frequent kidney stones, frequent vomiting, and that
has all made him unhappy. Which medication is likely to elevate his
plasma Calcium?
A. ACE inhibitor
B. HCTZ
C.
Metoprolol
D. Atorvastatin
Answer:
The correct answer is B) HCTZ. This is one of those
medications to remember, because it’s a commonly used first line
antihypertensive agent that we give to most patients presenting with
high blood pressure. Remember, HCTZ, Lithium, Vitamin A, Vitamin D,
and Milk Alkali syndrome are the ones to remember for exogenous causes
of hypercalcemia. A quick mnemonic to remember the symptoms of
hypercalcemia is stones (kidney stones), bones (bone pain), groans
(constipation), psychiatric overtones (depression, anxiety, insomnia)
What are diagnostic criteria for: Rickets
Age: prepubescent children
Abnormal Mineralization: Yes
Decreased bone mass: No
Bone deformation: Yes
Duration: Permanent
Primary pathology: Vitamin D deficiency
What are diagnostic criteria for: Osteomalacia
Age: Adults
Abnormal Mineralization: Yes
Decreased bone mass: No
Bone deformation: No
Duration: Temporary, until corrective action is taken.
Primary pathology: vitamin D deficiency
What are diagnostic criteria for: Osteoporosis
Age: Elderly
Abnormal Mineralization: No
Decreased bone mass: Yes
Bone deformation: No
Duration: Temporary, until corrective action is taken.
Primary pathology: Calcium deficiency, Sunlight deficiency Weight-bearing, activity deficiency
What is the difference between osmolarity and osmolality?
Osmolarity of volume based (osmoles/L) and osmolality is weight based (osmoles/kg)
Because most of human physiology is water-based and 1 L of water is roughly 1kg, these numbers are fairly similar when we’re talking about HUMAN PHYSIOLOGY. Outside of human physiology with other substances, they can vary drastically
What K levels you expect in someone with chronic hyponatremia?
Low levels (<3.5 mmol/L) because the kidneys are trying desperately to hold on to any Na that it comes in contact with, at the expense of K.
What pathology will cause all electrolytes to be elevated?
Dehydration
Bone matrix formation has what effect on children’s serum Ca and Mg levels?
Serum Ca and Mg are both slightly higher in children
What is the most abundant extracellular anion?
Chloride
A patient with diabetes has an elevated anion gap. What does this tell you about the current state of his disease?
Our patient is in diabetic ketoacidosis, undergoing anaerobic glycolysis, and producing lactic acid
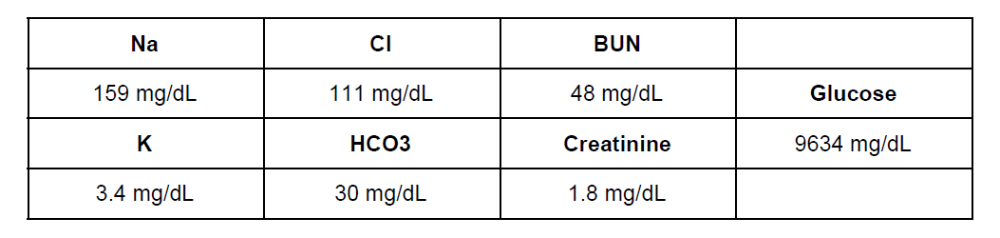
What is the underlying cause for all of her electrolyte abnormalities
is:
A. Ketoacidosis
B. Metabolic alkalosis
C. Renal
failure
D. Sodium ingestion
The correct answer is A) Ketoacidosis. She is likely a Type 1 diabetic, her anion gap is 159 - (111+30) = 18, which is mildly elevated so we must have caught it early. She has dehydration because she urinated so many times on the hike and the glucose is causing an osmotic diuresis. She has a low potassium level because her kidneys are attempting to retain all the sodium that they can to maintain her blood pressure, but that is in exchange for the potassium. Her potassium situation is actually more significant that this because of the potassium shift in acidosis. The extracellular hydrogen ions are exchanged for intracellular potassium because the cells want to help the body to handle the extra acid. Upon correction of the acidosis, her potassium may drop below 2 mg/dL. She has prerenal failure, which is consistent with dehydration. BUN/Cr ratio is ~27, which also is not too bad yet, so we’ve caught it before lots of bad things can happen.
A 56-year-old male presents to the clinic with vague abdominal pain and hypertension (153/105). The physician asks him first about his lifestyle which reveals that he eats out 4 times a week, does not exercise, and has had high blood pressure even when he did those things. He begins to make food at home more often, limit his sodium intake, and exercise. 3 months later, he returns to the doctor and his values don’t look any better, despite his lifestyle changes. The astute physician orders a serum aldosterone just on a hunch that he might have an adrenal tumor. The aldosterone comes back elevated. What laboratory values would you expect to see in Primary Hyperaldosteronism (Conn Syndrome)?
A. Na - 109 mg/dL K - 7.6 mg/dL Cr - 1.8 mg/dL
B. Na -
119 mg/dL K - 10.1 mg/dL Cr - 4.3 mg/dL
C. Na - 134 mg/dL K -
5.4 mg/dL Cr - 0.9 mg/dL
D. Na - 164 mg/dL K - 3.1 mg/dL Cr -
1.1 mg/dL
The correct answer is D) Na - 164 mg/dL K - 3.1 mg/dL Cr - 1.1 mg/dL Remember, aldosterone is the hormone that retains sodium in exchange for potassium. If you have a lot of aldosterone in your blood, then you’re going to have a very high sodium and a low potassium. The Creatinine value is not significantly elevated because of this pathology.
How will you recognize mercury poisoning on the registry exam?
The patient will have to have some sort of exposure, and will almost always have neurologic side effects
How will you differentiate the symptoms of Lead poisoning from Mercury poisoning on the Registry exam?
Answer: It’s going to be very hard to do that, as both heavy metals cause similar constellations of symptoms. The key to understanding the cause will be in the question stem, if they mention either Mercury or Lead, or I want you to think of Lead poisoning if they mention plasma aminolevulinic acid, whole blood zinc protoporphyrin, or free erythrocyte protoporphyrin.
What is the most predominant side effect of Lead poisoning?
Neurologic effects like behavioral changes, headaches, clumsiness
What are the expected total Iron, TIBC, Percent saturation, transferrin, and ferritin values for hemochromatosis?
Serum Iron ↑ TIBC ↓ % Sat ↑ Transferrin ↓ Ferritin
What are the two tests used to assess Copper?
Total Copper and Ceruloplasmin
What is the most likely method of analysis for Arsenic in the medical laboratory?
mass spectrometry
A premature male newborn was seen to have anemia on his initial labs.
The plan is for him to
be purely breastfed. A test is ordered
which is performed via mass spectrometry. It was also
noted that
the infant’s Ceruloplasmin levels were very low. What trace element is
deficient?
A. Arsenic
B. Copper
C. Iron
D. Lead
The correct answer is B) Copper. This deficiency is only commonly seen in premature infants and adults with malabsorptive pathologies. The presentation would include anemia, hepatosplenomegaly, and osteoporosis. Knowledge of this will help you understand the pathologies presented to you on the Registry exam, so you can get the right answer.
A patient of European ancestry comes to the physician complaining of
heart palpitations.
After life-threatening causes have been ruled
out, the primary care physician orders a Total
Serum Iron level,
Ferritin, Transferrin, TIBC, and % Saturation to assess for iron
levels because
his face and skin had turned a little darker
recently. The Serum Iron comes back as elevated, TIBC is decreased,
and % Saturation is increased. The Ferritin and Transferrin levels
were left off the report. What would you expect the results to be?
A. Transferrin ↑ Ferritin ↓
B. Transferrin ↑ Ferritin
↑
C. Transferrin ↓ Ferritin ↓
D. Transferrin ↓ Ferritin ↑
Transferrin ↓ Ferritin ↑
In a state of Iron excess, like
hereditary hemochromatosis, the body already has a lot of Iron, so it
will likely downregulate the amount of transferrin because it doesn’t
want to transport extra iron. The US Preventative Task Force has found
insufficient evidence to
recommend for or against using
Transferrin as a screening test for Hereditary Hemochromatosis. An
increased Ferritin level could just indicate Iron overload. A specific
test for Hereditary Hemochromatosis is demonstrating a genetic
mutation in the HFE gene.
A 37-year-old Female from Uzbekistan is beginning to complain of
numbness and tingling in her hands and feet. She is also experiencing
hair loss and mild anemia. Her husband makes his own bullets to
acquire dinner. She has been diagnosed with skin cancer at the young
age of 35. What trace element is the likely cause for these
symptoms?
A. Arsenic
B. Copper
C. Lead
D. Mercury
Arsenic causes a whole slew of symptoms, but primarily gastrointestinal symptoms at low doses. Also hematopoietic, neurologic, renal, and hepatic deficiencies. Remember that chronic exposure to this compound can predispose one to skin, liver, lung, bladder, and kidney cancers
What do we use to assess protein nutrition in patients?
Albumin, Transthyretin, Transferrin, CRP, or the Nitrogen Balance equation
What is the physiologic action for vitamin: A
Stored in the liver, important in vision, immune function, and growth; deficiency can lead to blindness
What is the physiologic action for vitamin: D
Synthesized in sequential steps in the skin, liver, and kidney, important in calcium homeostasis, associated with sun exposure; deficiency leads to osteomalacia
What is the physiologic action for vitamin: E
Prevents oxidative stress that cause aging and diseases like hemolytic anemia
What is the physiologic action for vitamin: K
Synthesized by intestinal bacteria and absorbed from leafy green vegetables; deficiency leads to prolonged bleeding
What vitamins are found to be deficient if the patient has fat malabsorption in the gut?
Answer: If a patient has fat malabsorption, then every one of these vitamins can be decreased. It usually takes a while for the symptoms to show up because we have a few months worth of supply stored in our fatty tissues.
A 37-year-old male is taking oral antibiotics for pneumonia that just won’t go away.
Answer: If a patient is taking long-term antibiotics, these can decrease the number of good intestinal bacteria which synthesize about half of your body’s Vitamin K which will cause you to be deficient
Vitamin K is important in what physiologic process?
Coagulation, in German, it’s spelled Koagulation
Function is to promote blood coagulation: for the coaguation factors 7,9, and 2
Trick 7, 92, blood coagulates for you
What is the effect and deficiency for vitamin: Thiamine (B1)
Effect: Coenzyme in decarboxylation
of
carbohydrates and branched-chain
amino acid pathways
Deficiency: Alcoholism, Causes beriberi
What is the effect and deficiency for vitamin: Riboflavin (B2)
Effect: Various oxidation-reduction reactions
Deficiency: Nutritional
deficiencies,
alcoholism, chronic malabsorption
What is the effect and deficiency for vitamin: Pyridoxine (B6)
Effect: Numerous functions
Deficiency: Uremia, malabsorption,
malignancies,
or alcoholism
Causes hyperhomocysteinemia
What is the effect and deficiency for vitamin: Niacin
Effect: Formation of reducing substances
NADH
and NADPH
Deficiency: Alcoholism, Causes Pellagra
What is the effect and deficiency for vitamin: Folate
Effect: Coenzymes in various one-carbon
transfer reactions
Deficiency: Adolescence, lactation,
or
alcoholism Causes megaloblastic anemia
without neurologic effects
What is the effect and deficiency for vitamin: Cobalamin (B12)
Effect: Coenzyme necessary for hematopoiesis
Deficiency: Pernicious anemia, strict
vegan
diet, tapeworm, or malabsorption Causes megaloblastic
anemia with neurologic effects
What is the effect and deficiency for vitamin: Ascorbic Acid (C)
Effect: Stabilization of collagen, cross-linking of tyrosine to
catecholamines
Deficiency: Deficiency seen in low fruit and vegetable intake, known as Scurvy Excess Vitamin C is rare because it is excreted in the urine
What is the most common causes of a deficiency in water-soluble vitamins?
Either low intake (malnutrition) or alcoholism
What mineral is likely deficient in the case of refractive hypocalcemia that will not correct?
Magnesium
A 47-year-old male who immigrated from South America is experiencing fatigue and a diminishing sense of touch in his fingers. He has also been complaining recently of a diminished sense of balance. The physician immediately recognizes the this person may have immigrated with Diphyllobothrium latum in his intestines. What vitamin would be deficient in this type of infection?
A. A
B. B12
C. C
D. D
Vitamin B12. Diphyllobothrium latum is a voracious tapeworm with an appetite for Vitamin B12. This will cause an infected individual to become deficient rather quickly. Symptoms of Vitamin B12 deficiency are neuropathies (i.e. loss of sense of balance, and sense of touch), and megaloblastic anemia. The physician would likely order a vitamin B12 level, CBC, and Folate level. The symptomatic difference between Folate and Vitamin B12 deficiencies is that Folate only produces megaloblastic anemia without neurologic effects.
2. At 6 am, a 69-year-old female is found on a park bench one night in the middle of winter with just a T-shirt on. She is slurring her words, but has reportedly been out since 2 am. A look back in police records show that she has been picked up for similar episodes about 35 times in the past 5 years. What water-soluble vitamins do you suspect her to be deficient in?
A. B1
B. B2
C. B12
D. C
all of the above. Sorry I didn’t add that option on purpose, because I wanted you to think about the probabilities of each of these vitamins individually. B1 and B2 are the most likely vitamins to be deficient in this scenario of chronic alcoholism. Vitamin C is also dependent upon diet, and unless she has no access to any citrus or vegetables, it would be quite rare for people who are not stuck on a boat at sea to come down with Scurvy (Vitamin C deficiency). Vitamin B12 is a horse of a different color. Yes, it is water soluble, but we have stores in the liver that can last us for years at a time. So, one has to be deficient in B12 for many years before symptoms begin to arise
A 58-year-old female is taking over-the-counter supplements to improve her vision and prevent skin aging to look youthful. She has been taking 700 IU/day. What vitamin is she likely taking?
A. A
B. B1
C. C
D. D
E. K
The correct answer is A) Vitamin A. Vitamin A primarily enhances vision, but is also is important in immune function, and growth. It has been found to decrease peroxidation of lipids, which will enhance a youthful appearance. Caution should be taken because too much of a good thing isn’t always good. Hypervitaminosis A can occur and cause, ironically, blurry vision, sun sensitivity, dry rough skin, hair loss, and confusion. Not exactly the intended effects of the supplement. This is one of the many reasons why patients should always discuss their supplement usage with their physicians
What are the fat soluble vitamins?
Vitamin A
Vitamin D
Vitamin E
Vitamin K
Memorize ADEK, or KADE, or DEAK or something similar
What vitamins does the liver store?
offers a place for storage for vitamins A, B12, D, E, K, and the minerals Iron, and Copper
Which vitamin does Erythrocytes store?
Thiamine
What do you need to know about vitamin A?
- AKA Carotenoid,
What is the pathology of Vitamin D (cholecalciferol) in the human body.
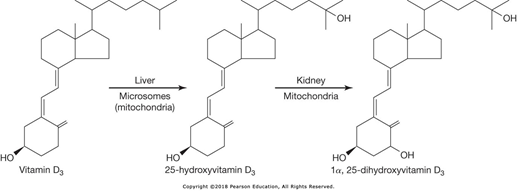
- Vitamin D is produced in the skin by sunlight
- Exposed to 25-hydroxylase in the liver
- Exposed to 1-hydroxylase in the kidney
- Active product is 1,25-dihydroxy Vitamin D
What do you need to know about vitamin D?
- Increases total body Calcium, necessary for bone health
- Associated with rickets in children
- Vitamin D deficiency commonly seen in individuals who live at higher latitudes and rarely venture outside
- Hypervitaminosis D is only present with mega doses of Vitamin D supplements
What do you need to know about vitamin K?
- Absorption is in the small intestine
- Deficiencies can occur in malabsorption, bile duct obstruction, pancreatitis, or liver failure
- Newborns usually need a shot of vitamin K to promote clotting in the first few days
- Measured using HPLC with electrochemical or fluorometric detection
What do you need to know about vitamin C?
- Antioxidant activity, iron absorption, biosynthesis of carnitine, and conversion of dopamine to norepinephrine
- Symptoms are bleeding, petechiae, Waterhouse-Friedrichsen Syndrome (bloody adrenal glands with necrosis)
- Hypervitaminosis C may cause ALT, LD, and uric acid to be elevated, and may form kidney stones
- Assessment by HPLC or GC/MS
What do you need to know about Thiamine (B1)?
- Dry beriberi - primarily neurologic symptoms
- Wet beriberi - cardiovascular symptoms resulting from a thiamine-deficient pump
- Wernike-Korsakoff syndrome - long-term alcoholism, horizontal nystagmus, cerebral ataxia, and mental impairment
- Must supplement Thiamine to avoid refeeding syndrome and death when feeding starved individuals
Measured by HPLC
What do you need to know about Cyanocobalamin (B12) ?
- Allows for DNA synthesis and cell division to take place
- Pernicious Anemia - Megaloblastic anemia due to impaired DNA synthesis and B12 deficiency
- Indirect measurement by Methylmalonic Acid (MMA), plasma homocysteine, deoxyuridine suppression, or the vitamin B12 absorption test
- Direct measurement by competitive binding protein assay using Co57 labeled cobalamin, immunometric methods, or chemiluminescent immunoassays
- Requires Intrinsic factor for absorption in the ileum
What do you need to know about Folic Acid (B9) AKA folate?
- Process one-carbon units
- Major manifestation of deficiency is megaloblastic anemia, but without neurologic abnormalities (as opposed to Vitamin B12 deficiency)
- Evaluate by chemiluminescence or enzymatic methods
- Given to pregnant women to avoid neural tube defects in the fetus
What are reference values for some nutritional deficiencies?
Serum Albumin < 3.0 g/dL suggests possible malnutrition (Normal 3.5-5.0 g/dL)
Blood Urea Nitrogen <6.0 mg/dL suggests inadequate protein intake
HPLC for specific identification of vitamins
Which organs maintain calcium homeostasis?
1.Small intestine
2.Kidneys/Liver
3.Skeleton
What is the role of PTH in Ca homeostasis?
primary job is to increase circulating Ca ++
- Primary purpose is to increase the ionized Ca++ concentration in the blood
- A primary regulator of plasma Ca++ concentration
- Increases bone resorption (destruction, harvesting Calcium)
- Increases kidney reabsorption of Ca
- Removes PO4 2- from the blood
- Increases intestinal absorption of Ca
What is the role of Vitamin D in Ca homeostasis?
- primary job is to increase total body Ca ++
What is the role of Calcitonin in Ca homeostasis?
- primary job is to decrease circulating Ca++
What is the difference between the activities of Calcitonin, Parathyroid Hormone, and Vitamin D on bones?
Calciton”IN” and Vitamin D put calcium INto the bones for storage, ParathyRoID Hormone gets RID of the Calcium in the bones
What are six causes of Hypocalcemia?
1.PTH Deficiency
2.PTH Resistance
3.Vitamin D Deficiency
4.Deficiencies in Bone Mineralization
5.Renal
6.Metastatic
What are the mechanisms of Hypercalcemia Malignancy?
1.Tumors release PTH-related Peptide
2.Osteolytic metastases
What happens to the total calcium and PTH with the following disorder: Secondary Hyperparathyroidism
Total calcium: Increased
PTH: Increased
What happens to the total calcium and PTH with the following disorder: Hypercalcemia of Malignancy
Total calcium: Increased
PTH: Decreased
What happens to the total calcium and PTH with the following disorder: Hypoparathyroidism
Total calcium: Decreased
PTH: Decreased
What is the definition of Electrolyte?
- small ionizable constituents in the body
What are the intracellular ions?
potassium, phosphate, calcium, magnesium
What are the Extracellular ions?
sodium, chloride, bicarbonate
What is the Anion gap?
the normal gap between measured cations and measured anions, the difference represents the unmeasured anions
What are the trace elements you need to know?
the ones that we will worry about are iron, copper, and zinc
What should you know about Compartmentalization of electrolytes?
Intracellular fluid has a substantially higher level of potassium, proteins, organic phosphates and acids.
Notes type of pressure:
Oncotic pressure
Osmotic pressure
Hydrostatic pressure
How does ANP/BNP effect Homeostatic regulation?
Natriuretic effects released from the cardiac ventricles
How does Renin-Angiotensin- effect Homeostatic regulation?
Aldosterone System (RAAS):is a critical regulator of blood volume, electrolyte balance, and systemic vascular resistance
released from the kidney in response to low O2 saturation in renal blood
How does Angiotensin effect Homeostatic regulation?
released as angiotensinogen from liver, becomes ATI, then is converted to ATII in lungs, potent vasoconstrictor
How does Aldosterone effect Homeostatic regulation?
- secreted by adrenal glands, increase Na+ reabsorption in exchange for K+ and decrease H2O excretion
What is the formula for osmolarity?
(2*Na) + (BUN/2.8) + (Glucose/18) =
Note: Normal Range: 275-295 mOsm/L
What is the normal osmolar Gap?
5-10 mOsm/kg
note: Anything above 5-10 indicates that there are osmotically active substances which differ from the ones we include in the calculation (e.g. Na, BUN, Glucose)
What values should you be aware of for Syndrome of Inappropriate ADH Secretion (SIADH)?
Expect very low plasma osmolality (e.g. <275 mOsm/kg)
Expect very high urine osmolality (>100 mOsm/kg)
What are some common ISE?
1.Sodium
2.Potassium “valinomycin”,
3.Chloride
What are the values that should be known for natremia?
- <135 mEq/L
note: Can cause hypovolemia, altered mental status, orthostatic hypotension,
- > 150 mEq/Lnote: generally caused by dehydration, burns, or after hypertonic saline
What are the values that should be known for kalemia?
< 3.5 mEq/L, can be caused by K+ shift in treated acidosis, polyuria, diarrhea, blood dilution, mild dehydration, laboratory error, or sample collection error. This can cause low a K sample
> 6.1 mEq/L, can be caused by hypoaldosteronism, extreme dehydration, hemolysis, tourniquet during collection, laboratory error, or sample collection error
Critical Values - <3 mEq/L and >8 mEq/L
What are the values that should be known for chloremia?
>108 mEq/L - Intake of Cl- exceeds the output and primarily caused by dehydration and other causes of Na+ retention. REMEMBER the chloride shifts out of cells in alkalosis
< 97 mEq/L - Output of Cl- exceeds intake caused by the same list as hyponatremia with an important exception, metabolic alkalosis. This is due to GI loss of Cl- but not Na+. REMEMBER the chloride shifts into cells in acidosis
What is Wilson disease?
Ceruloplasmin levels are very low , but there is a lot of copper deposition in the tissues. This relationship is similar to Iron and Transferrin
The deficiency is in a Copper transporter in the liver which removes the Copper from circulation into the bile, so these patients can’t get rid of their Copper!
Treatment is intravenous British Anti-Lewisite (BAL), D-penicillamine, or both because they will chelate the copper
What are the two fundamental tests used to assess Copper?
Serum Copper and Serum Ceruloplasmin
Formula for calculating anion gap?
Anion Gap = Na - [Cl + HCO 3 ] Reference range = 7-16 mEq/L
What does the acronym MUDPILES stand for?
Common causes of metabolic acidosis with an elevated anion gap
- Methanol
- Uremia
- DKA
- Paracetamol
- Isoniazid
- Lactic Acidosis
- Ethanol
- Salicylates
What is freezing point depression used for?
for sweat chloride testing for Cystic Fibrosis in neonates we now used a PCR to find the gene.
17-year-old Male presents to the physician with abdominal cramping 2 hours after ingesting 12 glazed donuts. What is the full medical break down?
Test: Serum Na
Sample: Serum or Plasma
Method: Ion-Selective Electrode
Result: 121 mg/dL
Reference Range
(136-146 mg/dL)
Disease/Physiology: Lipemia
43-year-old Female with kidney stones, constipation, and multiple
fractures. Ultrasound of the thyroid reveals a completely
normal
thyroid. What is the full medical break down?
Test: Serum Calcium
Sample: Serum or Plasma
Method: Ion-Selective Electrode
Result: 11.3 mg/dL
Reference Range
(8.4-10.2 mg/dL)
Disease/Physiology: Primary, Hyperparathyroidism
68-year-old Male Vietnam veteran is feeling weak and tired all the time, CBC revealed macrocytic anemia. He mentions that he doesn't eat very much for breakfast, lunch, or dinner, but does drink liquor throughout the day
What is the full medical break down?
Test: Thiamine
Sample: Whole Blood
Method: Rapid HPLC
Result: 1.5 μg/dL
Reference Range
(2.5-7.5 ?g/dL)
Disease/Physiology: Chronic Alcoholism
29-year-old healthy asymptomatic Male presents to the clinic for his annual physical.
What is the full medical break down?
Test: Serum Potassium and Calcium
Sample: Serum or Plasma
Method: Ion-Selective Electrode
Result: K+ 8.4 mg/dL
Ca++ 5.2 mg/dL
(3.5-5.0
mg/dL and
8.4-10.2 mg/dL)
Disease/Physiology: Sample Collected In Potassium EDTA
20-year-old Female with longstanding diarrhea over the past 4 months and presents to her family medicine physician complaining of consistent bleeding from her lips. What is the full medical break down?
Test: PT/INR A prothrombin time (PT) test measures how long it takes for a clot to form in a blood sample. An INR (international normalized ratio) is a type of calculation based on PT test results
Sample: Citrated Plasma (Blue Top)
Method: PT/INR
Result: 32 sec
Reference Range
(11-13.1 sec)
Disease/Physiology: Vitamin K Dependent Coagulation Factor Deficiency
63-year-old Female gets her DEXA scan results back to measure her bone mineral density, they come back quite low. She spends most of her time in front of her computer working and eats microwaveable meals quickly so she can bet back to work.
What is the full medical break down?
Test: Calcium
Sample: Serum or Plasma
Method: Ion-Selective Electrode
Result: 7.8 mg/dL
Reference Range
(8.4-10.2 mg/dL)
Disease/Physiology: Osteopenia Secondary To Vitamin D Deficiency
How do we evaluate Iron status?
1. Serum Iron - specifically 3+ Iron bound to transferrin, NOT free
floating 2+ Iron
2. Total Iron Binding Capacity - theoretical
amount of iron that could be bound to all of the transferrin binding
sites available
3. Percent Saturation - it measures the ratio of
transferrin to bound iron
4. Transferrin - the transport protein
for Iron, increased in anemia, decreased in hemochromatosis
5.
Ferritin - the storage form of Iron