three chemical structures for unsaturated hydrocarbons
alkenes alkynes
What color changes did you observe when you brominated ethene? What type of reaction was that?
The solution experienced a color change to dark red
Write a chemical reaction for the conversion of benzene into bromobenzene by the reaction with bromine in the presence of iron (III) chloride. What type of reaction was that?
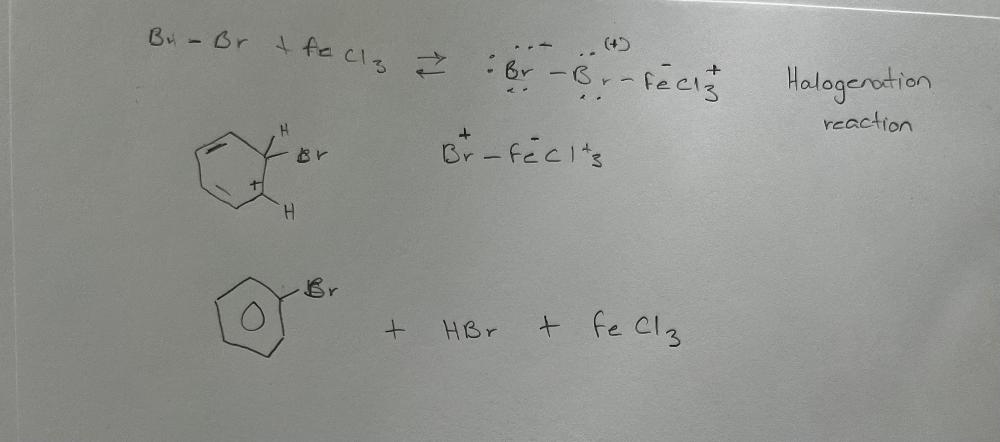
What happens when you react cyclohexene with bromine and cold potassium permanganate?
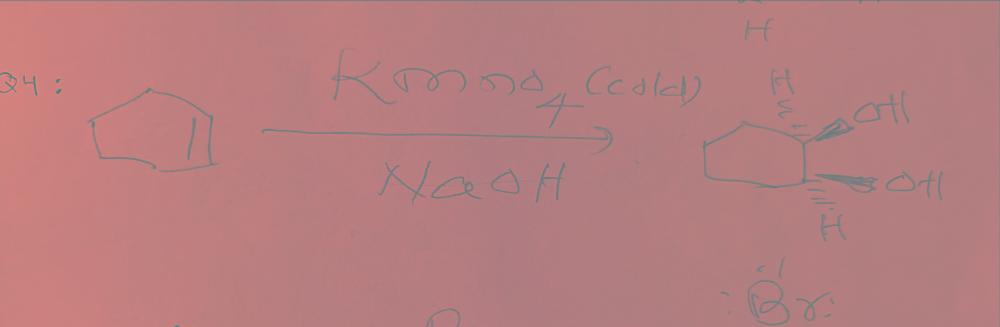
Write a chemical equation for the reaction of acetylene with 2 mols of bromine in the presence of a solvent carbon tetrachloride?
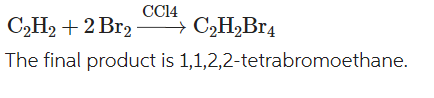
What is the main safety concern about hydrocarbons?
Hydrocarbons are highly flammable liquids and vapor. It is also fatal if swallowed.
Why should bromine be handled with care?
Bromine is corrosive and the dark vapor of bromine can irritate eyes.
Where should all reactions for this lab (reactions of hydrocarbons) take place.
It should take place in the fume hood.
product of reaction of given hydrocarbon and bromine

What visual observation would you expect if you ran the reaction of the above hydrocarbon with bromine?
When bromine is added to 2-butene, a light brown color would occur. After, the reaction is completed the brown color would completely fade (meaning the bromine was completely consumed by the alkene).
what precautions should one use when working with H2SO4 and H3PO4
Gloves, closed toed shoes, lab coat, goggles because it can burn skin.
Calculate the theoretical yield
...
when 2- butanol undergoes E1 dehydration, three alkenes are obtained. Draw the structures for these alkenes. Which is the most abundant.
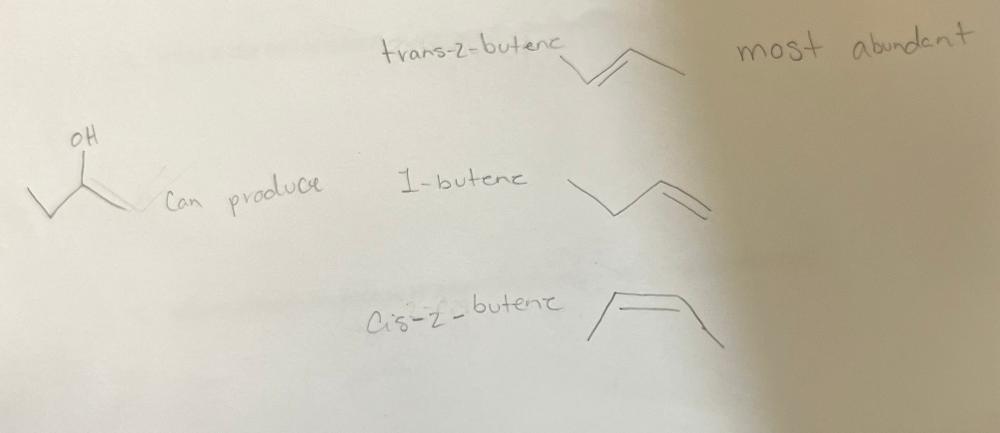
Most abundant is trans 2-butene
Write a chemical equation to convert cyclohexanol into cyclohexene in the presence of an acid catalyst.
C6H11+H3PO4----^C6H10+H2O
between ethanol and tert-butanol which alcohol dehydrates at much faster rate?
Tert-butanol will dehydrate faster because it is a tertiary alcohol.
What is the role of a catalyst during dehydration?
The role of a catalyst during dehydration is to drive the reaction in one direction only and speeds up the reaction.
Why acid catalysts such as HCl and HBr are not used during dehydration?
Because their conjugate bases are good nucleophiles that can cause a substitution reaction to occur.
What precautions should one use when working with potassium hydroxide?
Plastics and some rubber might be affected by the potassium hydroxide.
why is it important that the number of moles of 2 naphthoxide ion be the same as the number of moles of iodoethane used for the synthesis of nerolin?
We use the same number of moles for both because in the synthesis of nerolin the molar ration of naphthoxide to iodoethane is 1:1. This ensures a that no extra reactants are found after the reaction.
What effect would not properly drying the filtered nerolin have on your results?
This would affect your experimental and percentage yield.
Identify the nucleophile and leaving group for the reaction
2-naphthoxide acts as the nucleophile and Iodoethane as the leaving group.
Write a chemical equation for the formation of nerolin from 2-hydroxynaphthalene
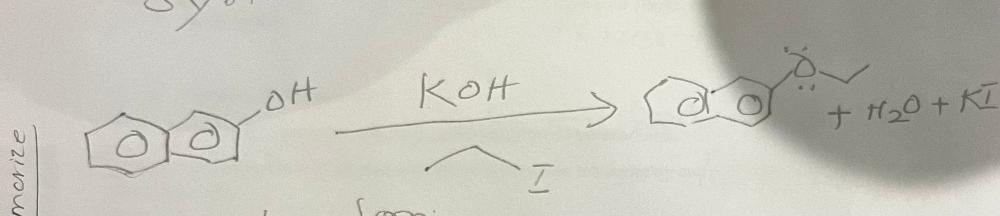
write a rational mechanism for the formation of nerolin
paper
What is nerolin used for
it is used for perfumes, as a stabilizer in powders.
calculate percent yield:
actual/theoretical*100
How do you know nerolin is pure:
melting point and solubility in alcohol. Does not dissolve in water.
What does sn1 mean
Substitution, nucleophilic and unimolecular
how to identify an SN1 reaction
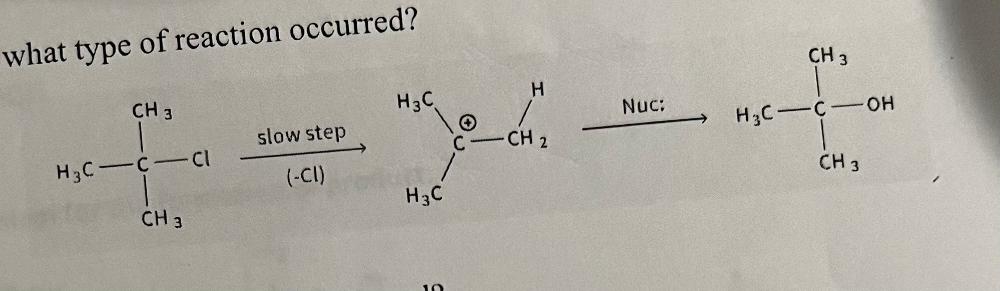
how to identify an E1 reaction
will have a leaving group followed by a carbocation intermediate and then the base will create an alkene
which of the alcohols named below will react fastest with HCl to form the corresponding alkyl chloride via an Sn1 pathway?
t-butyl alcohol . This is because it is able to form a tertiary carbocation when the leaving group is gone unlike other structures.
The rate of a first order chemical reaction is proportional to the concentration of electrophile
yes because the concentration and the rate of the first reaction are directly proportional.
Write a chemical equation for the conversion of tbutyl chloride with water
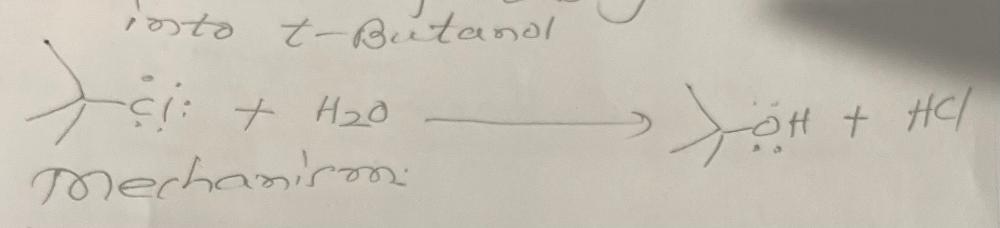
Write a rational mechanism for the formation of product
experiment 8 SN reaction
What happens when ethyl chloride is treated with NaOH
C2H2Cl+NaOH---^C2H5OH+NaCl