What is the definition of Oxidation?
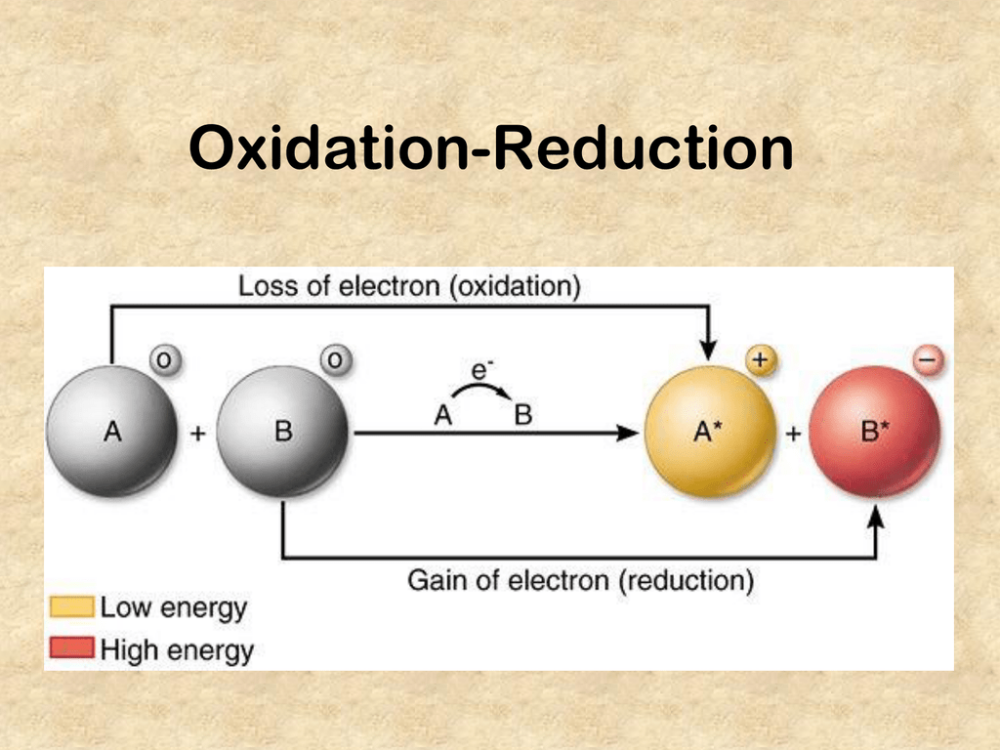
loss of electrons
What is the definition of Reduction?

gain of electrons
What is the definition of a redox reaction?
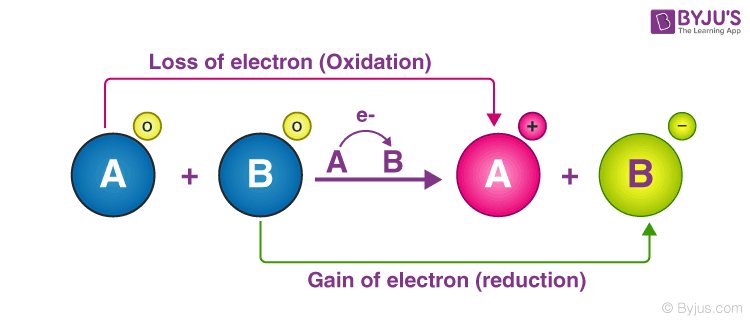
electrons are transferred from one species to another. A molecule is said to be oxidized when it loses electrons. It is reduced when it gains electrons.
What is the definition of oxidizing agent?
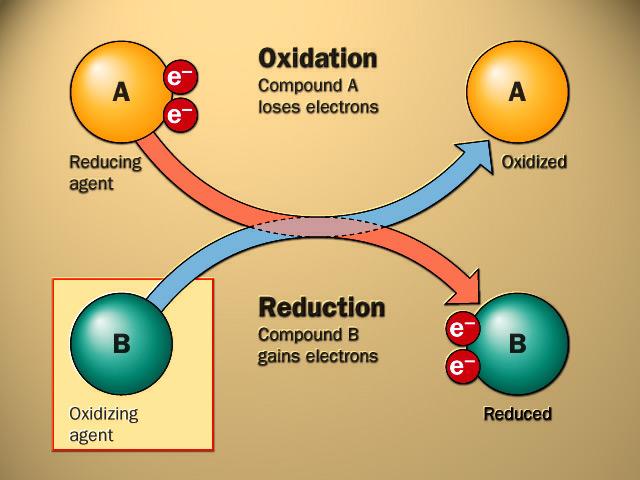
also called an oxidant, takes electrons from another substance and becomes reduced.
What is the definition of reducing agent?

also called a reductant, gives electrons to another substance and is oxidized in the process. In the reaction
What is the definition of coulombs?
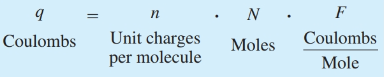
Electric charge (q)
What is the definition of Faraday constant?
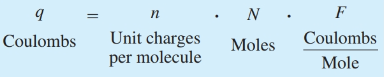
F=96 485.3321 s A / mol
What is the definition of Electric current?
(I) is the quantity of charge flowing each second past a point in an electric circuit.
What is the definition of ampere?
- which is a flow of one coulomb per second.
- The unit of current
What is the definition of an electrode?
a device to conduct electrons into or out of the chemicals involved in the redox reaction.
What is the definition of an electroactive species?
a molecule that can donate or accept electrons at an electrode.
What is the definition of Electrolysis?
is a chemical reaction in which we apply a voltage to drive a redox reaction that is not spontaneous (not energetically favorable) and would not otherwise occur.
What is the definition of electric potential?
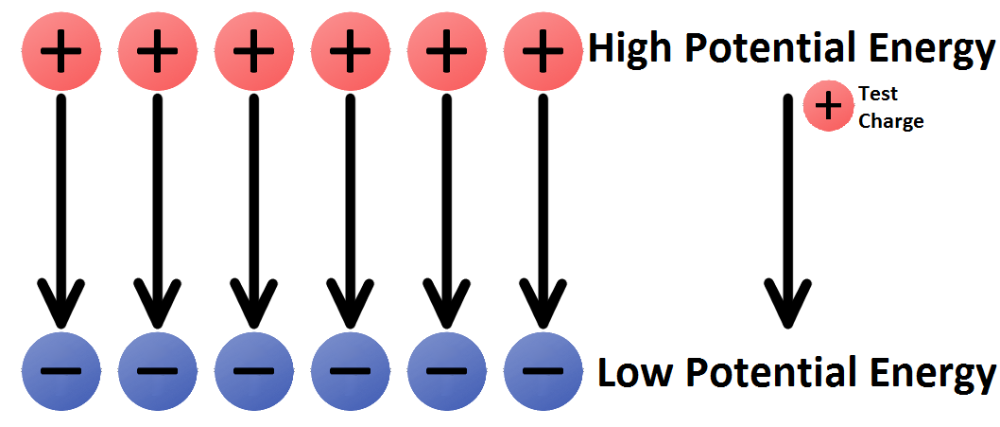
- The difference between two points measures the work that can be done (or is needed) when electrons move from one point to another.
- The greater the potential difference between points A and B, the more work can be done (or must be done) when electrons travel from point A to point B. Potential difference is measured in volts (V).
What is the definition of a spontaneous reaction?
it is energetically favorable for reactants to be converted into products. Energy released from the chemicals could be available as electrical energy.
What is the definition of a galvanic cell?
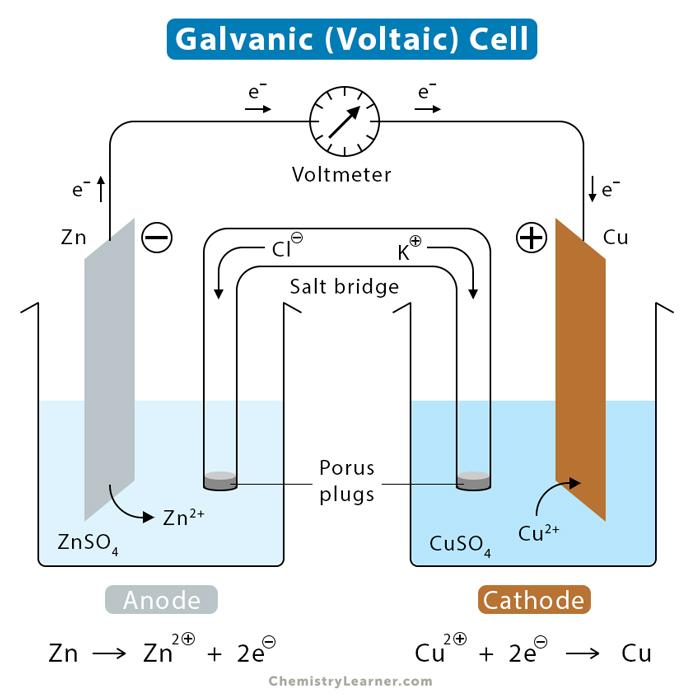
- a spontaneous chemical reaction generates electricity.
- an electrochemical cell that produces a potential difference by the conversion of chemical energy into electrical energy
What is the definition of a salt bridge?
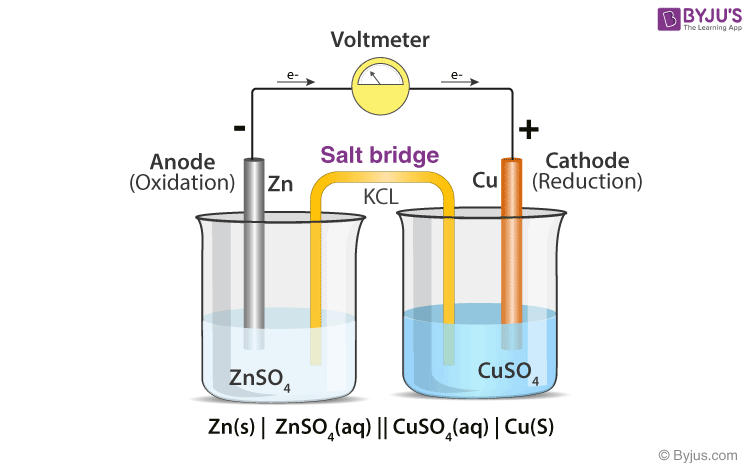
is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution.
What is the definition of a cathode?
electrode at which reduction occurs
What is the definition of an anode?

the electrode at which oxidation occurs
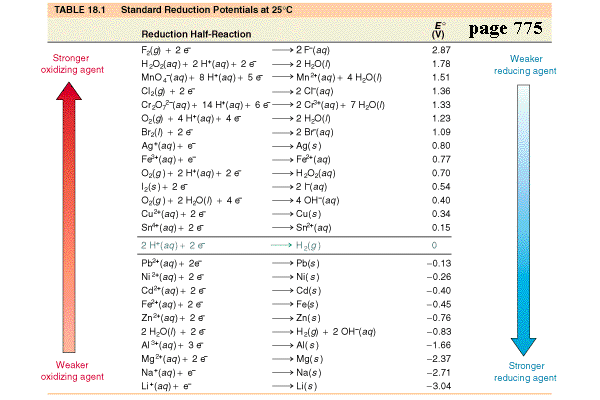
What is the definition of standard reduction potential (E°)?
- measures the tendency for a given chemical species to be reduced.
- The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced.
What is the definition of standard hydrogen electrode (S.H.E.)?
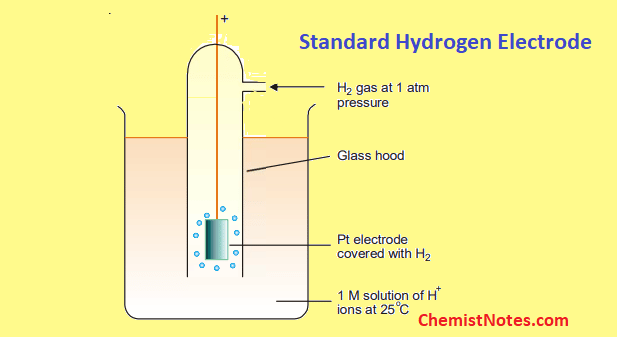
- an electrode that scientists use for reference on all half-cell potential reactions.
- The value of the standard electrode potential is zero, which forms the basis one needs to calculate cell potentials using different electrodes or different concentrations.
What is the definition of formal potential?
The potential for a cell containing a specified concentration of reagent other than 1 M
What is the definition of an Indicator electrode?
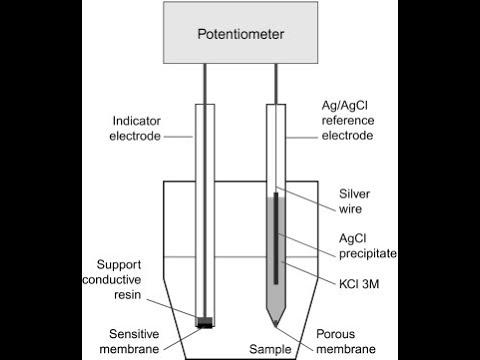
- responds to analyte concentration
- potential changes in order to measure things
What is the definition of a Reference electrode?

- maintains a fixed (reference) potential
- self contained
What is the definition of a silver-silver chloride electrode?
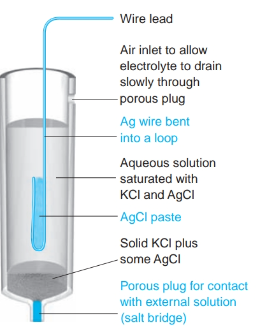
- an electrode where the metal (silver) is in contact with a slightly soluble salt (silver chloride), which in turn is in contact with a solution containing the common anion (chloride ion).
- The ions in the solid phase and those in the liquid phase are in equilibrium.
What is the definition of a saturated calomel electrode S.C.E.
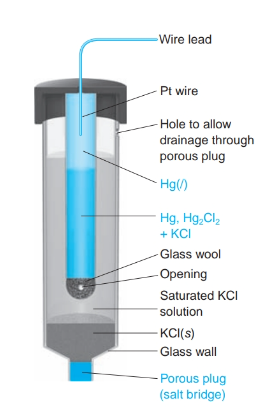
a reference electrode based on the reaction between elemental mercury and mercury (I) chloride.
*when saturated Cl- does not change with evaporating liquid.
What is the definition of a volt?
the SI unit of electromotive force, the difference of potential that would drive one ampere of current against one ohm resistance.
When is the double junction electrode used?
if you do not want the analyte and electrode to have direct contact.
What are some common indicator electrodes?
- Metal electrodes
- ion selective electrodes(I.S.E..)
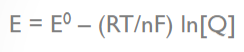
What does the variable "E" stand for in the Nernst equation?
Electromotive force of a cell
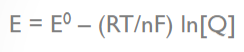
What does the variable "E0" stand for in the Nernst equation?
standard reduction potential
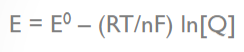
What does the variable "R" stand for in the Nernst equation?
molar gas constant
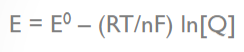
What does the variable "T" stand for in the Nernst equation?
temperature in degrees Kelvin
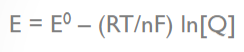
What does the variable "n" stand for in the Nernst equation?
number of moles of electrons transferred
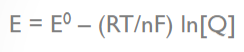
What does the variable "F" stand for in the Nernst equation?
Faraday’s constant (96,500 C/mol)
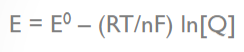
What does the variable "Q" stand for in the Nernst equation?
the reaction quotient (a function of the activities or concentrations of the chemical species involved)
What is the definition of electrolyte?
a substance that dissociates into ions in solution and acquires the capacity to conduct electrons
What is the definition of electrode potential?
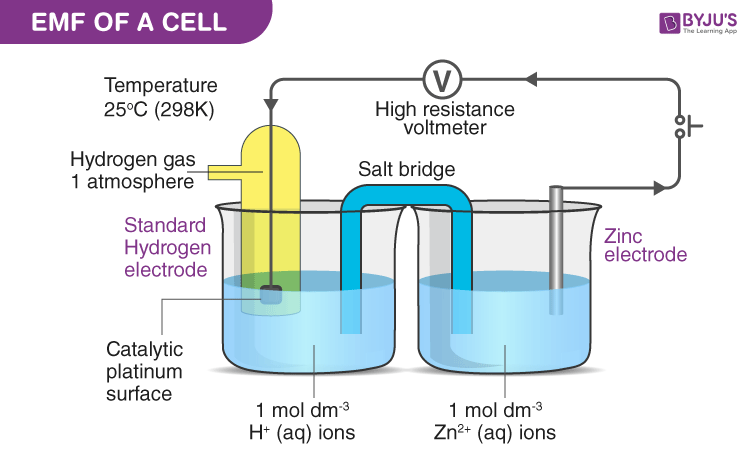
- The reduction potential for a redox couple measured
with
respect to a standard hydrogen electrode set at zero. - The electromotive force of an electrochemical cell.
What isthe standard state of an element?
- liquids: 1M concentration,
- gases: pressure 1 bar
- Temperature; 298 K(25°C )
What is the Nernst equation for?
o Calculate the voltage of an electrochemical cell
o Find the
concentration of one of the components in a system
What is the definition of ELECTROLYTIC CELL?
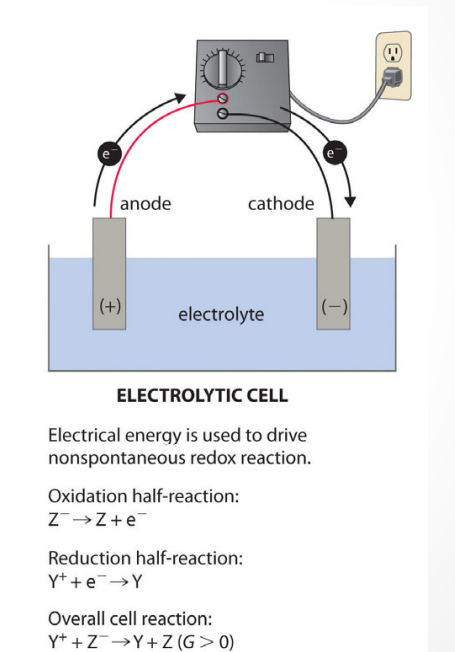
an electrochemical cell in which chemical reactions occur through the application of an external potential difference