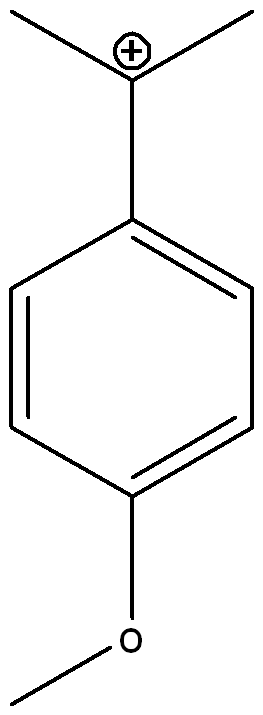
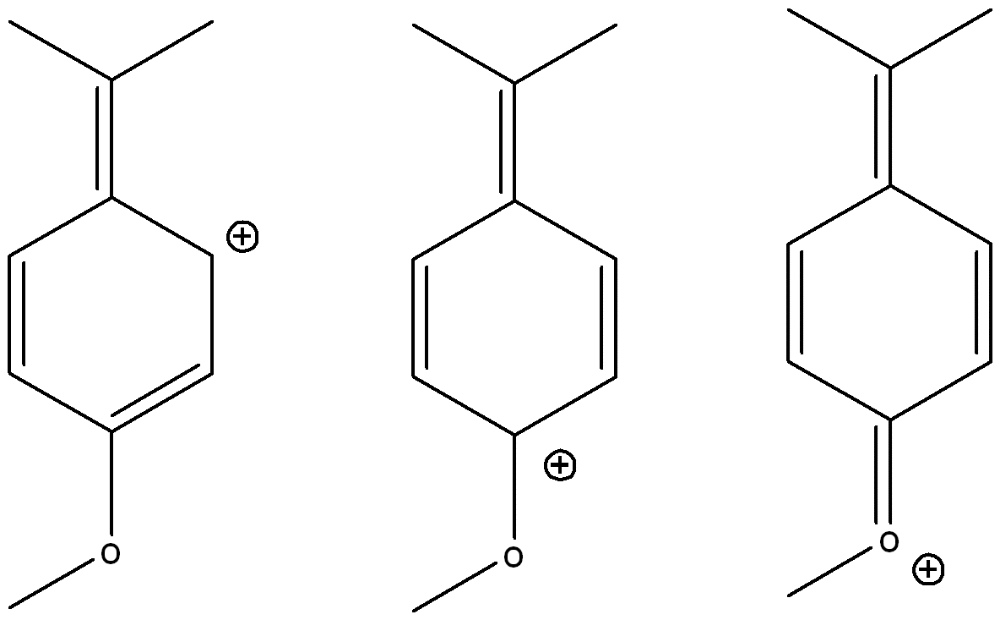
Draw three other possible resonance structures
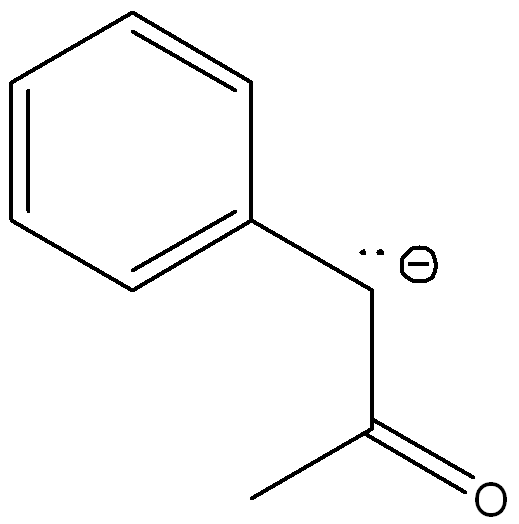
Draw another resonance structures and determine which resonance contributes the most to the resonance hybrid
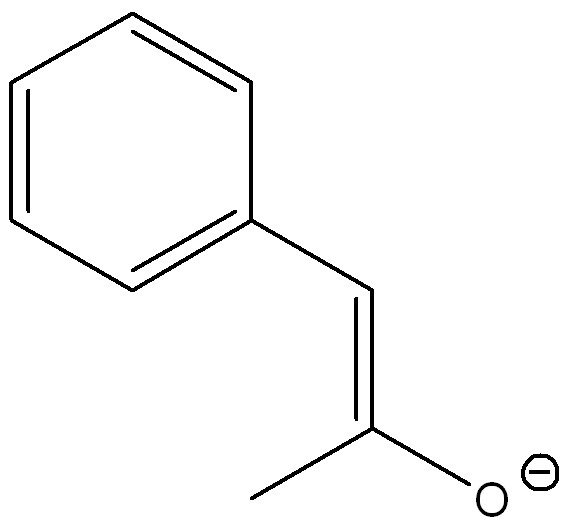
The second structure contributes more because the negative charge is on the more electronegative atom (oxygen)
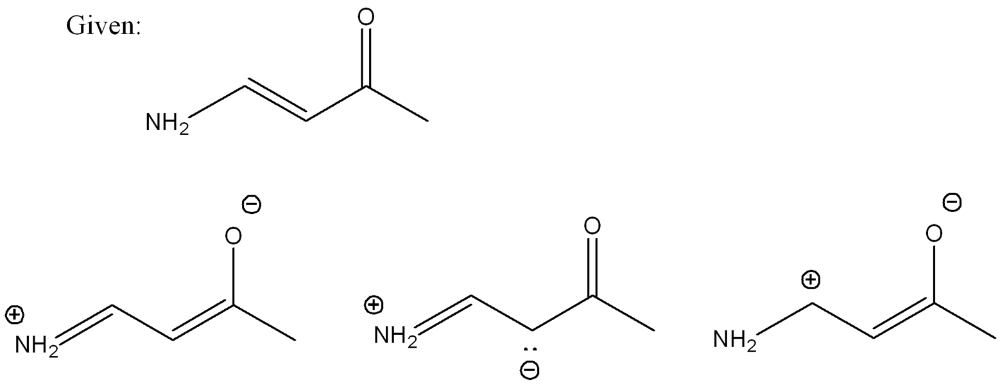
Determine which resonance structure contributes the most to the hybrid of this structure (out of the three options)
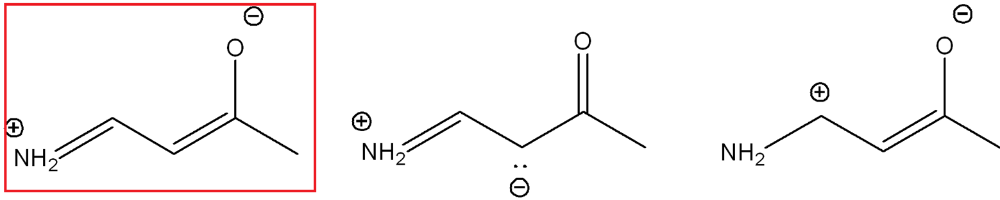
Draw a resonance structure that contributes more to the hybrid. Use arrow notations and show all formal charges.
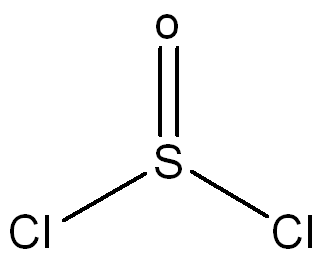
Draw a Lewis Structure for the following molecule SOCl2 and assign necessary charges.
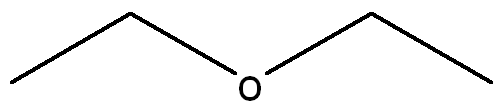
Draw a Lewis Structure for the following molecule CH3CH2OCH2CH3 and assign necessary charges.
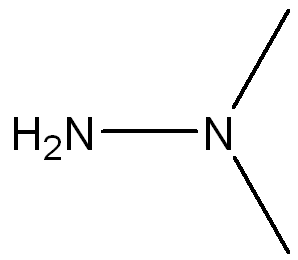
Draw a Lewis Structure for the following molecule (CH3)2NNH2 and assign necessary charges.
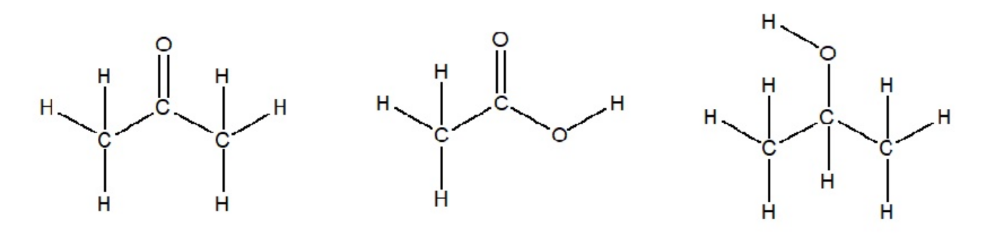
Determine the Lewis Structure for acetone (CH3COCH3), Acetic Acid (CH3COOH) and isopropanol ((CH3)2CHOH)
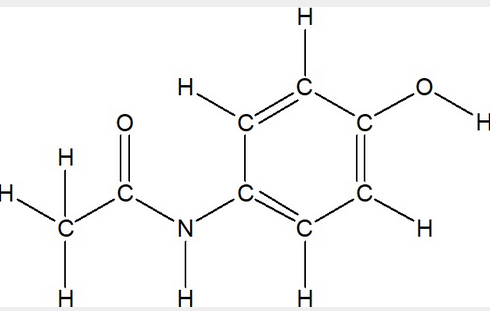
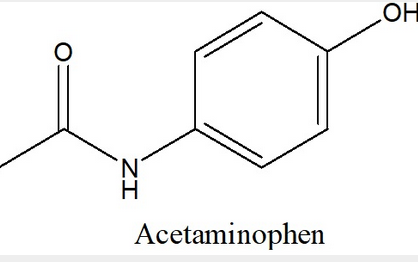
Convert the given Lewis Structure of Acetaminophen (Tylenol) into a Condensed Structure
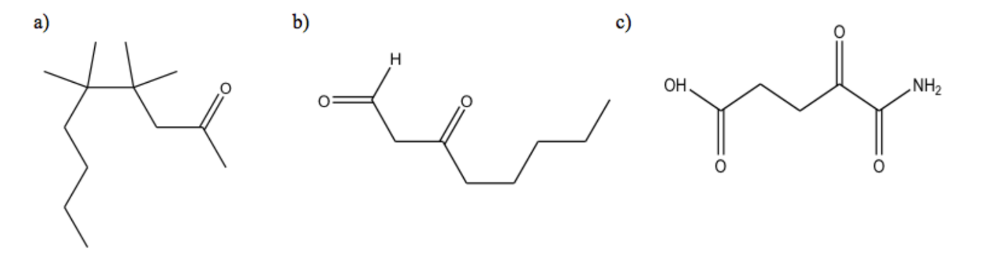
For the following compounds, give the chemical formula of the condensed structure
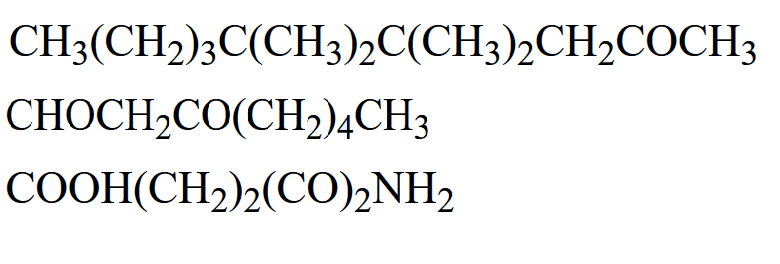
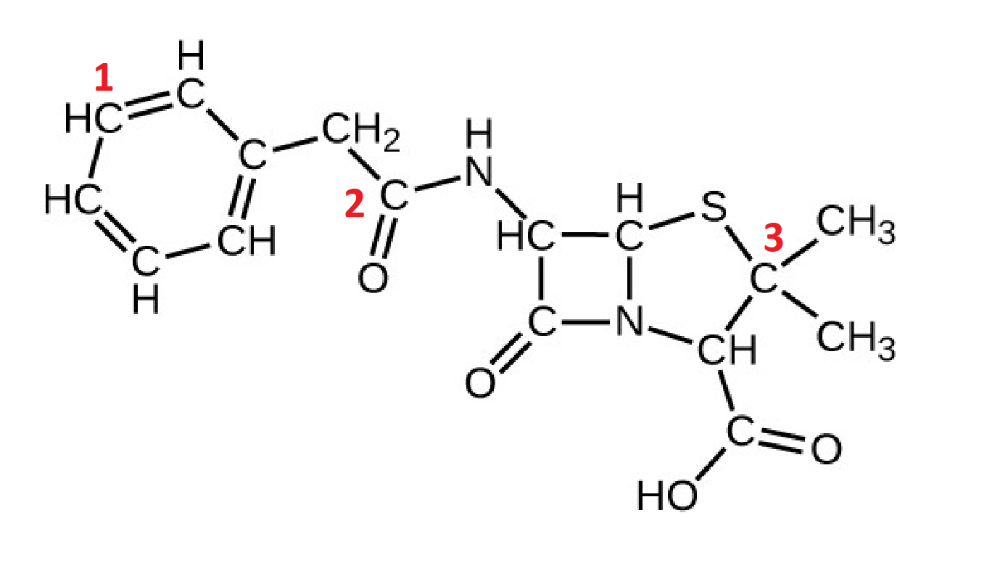
Identify the orbitals involved in the labeled carbons:
C1 and H
C2 and O
C3 and S
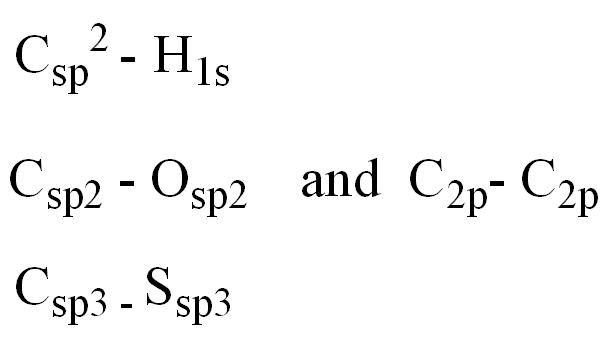

Determine the orbitals for the numbered atoms and bonds:
C1 and adjacent Carbon
N2 and H
C3 and adjacent Carbon
C and S4
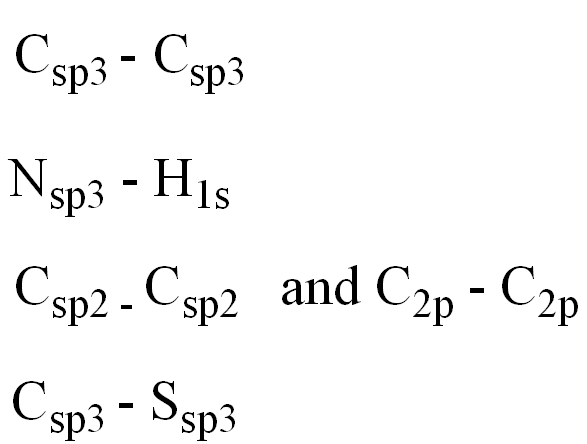

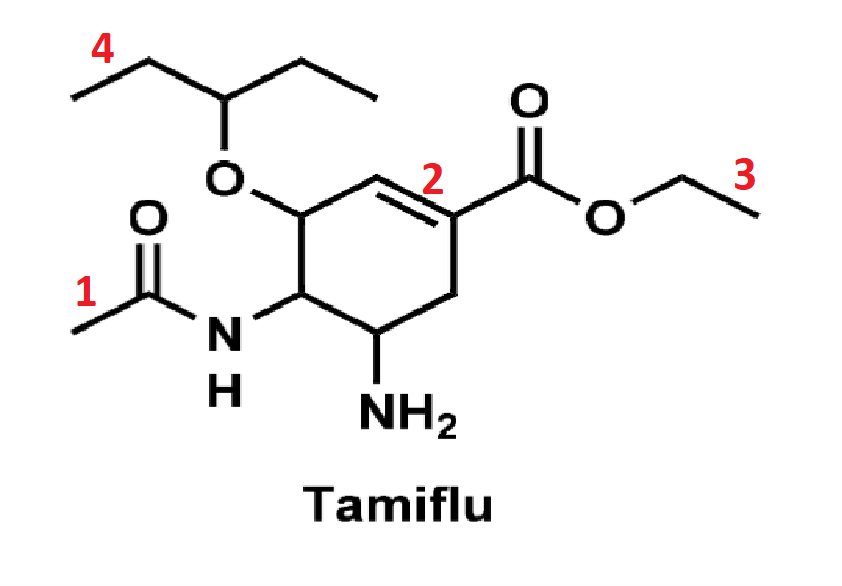
What is the hybridization and molecular geometry of each labeled atom?
1- sp3 hybridized and tetrahedral
2- sp2 hybridized and trigonal planar
3- sp2 hybridized and trigonal planar
Rank the following bonds in order of increasing strength
4 < 3 < 1 < 2