Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Chapter 1 - Structure and Bonding
front 1 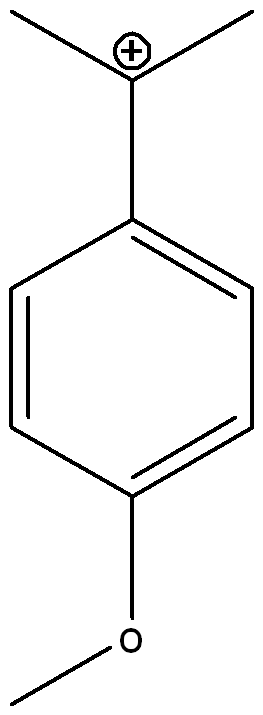 Draw three other possible resonance structures | back 1 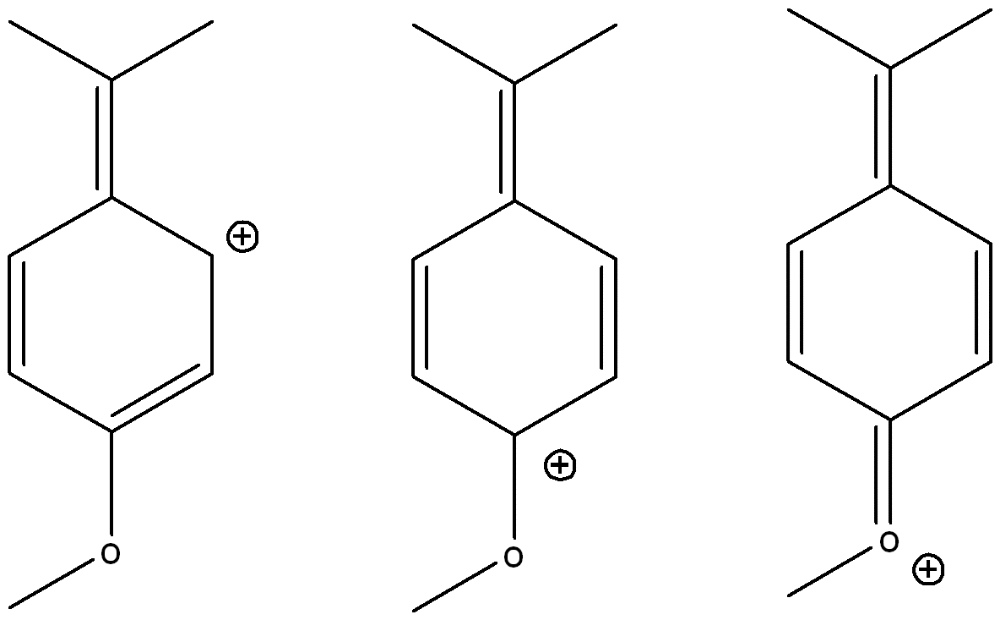 |
front 2 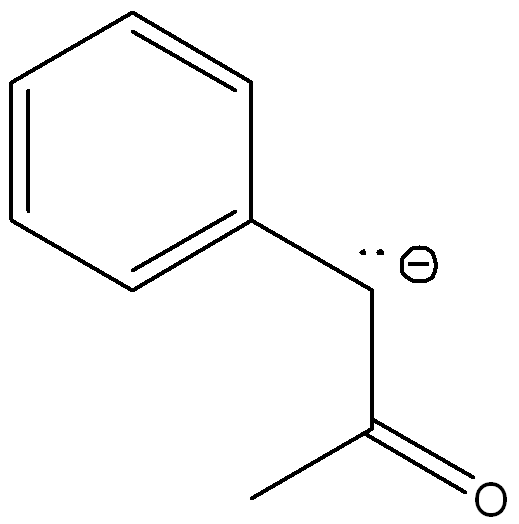 Draw another resonance structures and determine which resonance contributes the most to the resonance hybrid | back 2 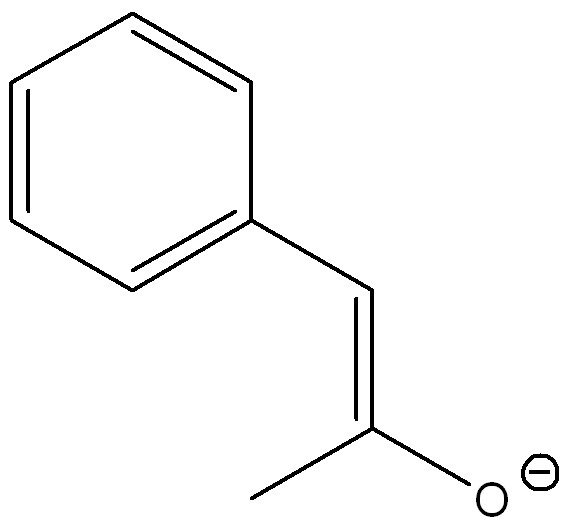 The second structure contributes more because the negative charge is on the more electronegative atom (oxygen) |
front 3 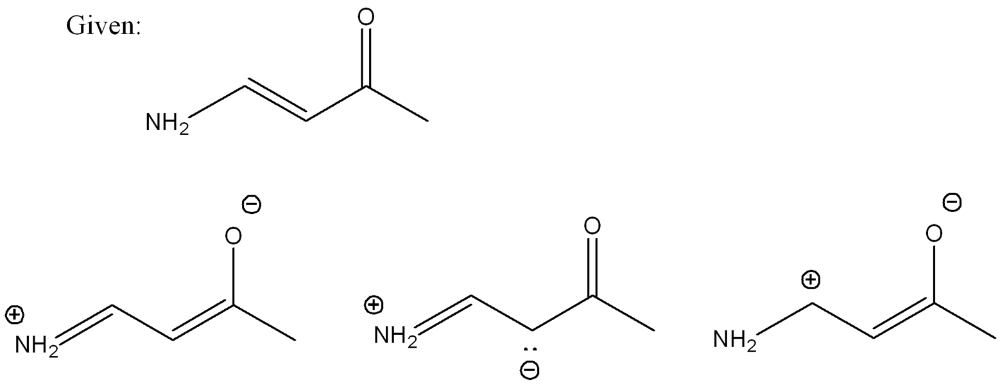 Determine which resonance structure contributes the most to the hybrid of this structure (out of the three options) | back 3 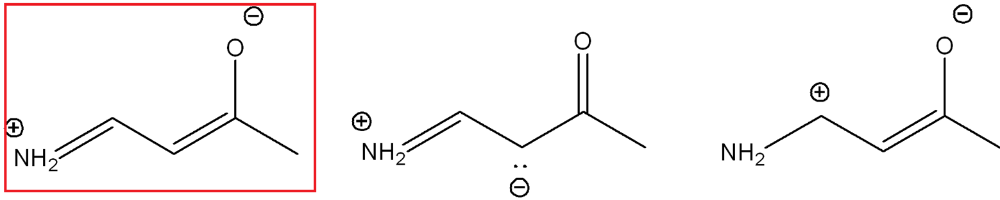 |
front 4 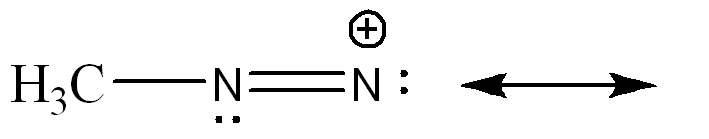 Draw a resonance structure that contributes more to the hybrid. Use arrow notations and show all formal charges. | back 4 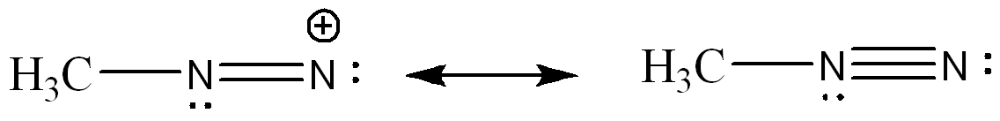 |
front 5 Draw a Lewis Structure for the following molecule SOCl2 and assign necessary charges. | back 5 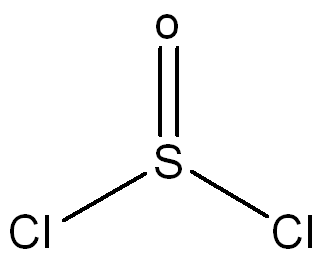 |
front 6 Draw a Lewis Structure for the following molecule CH3CH2OCH2CH3 and assign necessary charges. | back 6 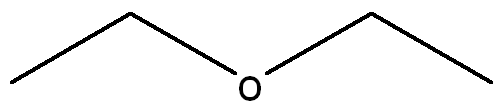 |
front 7 Draw a Lewis Structure for the following molecule (CH3)2NNH2 and assign necessary charges. | back 7 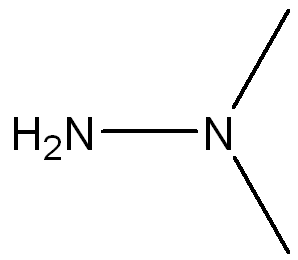 |
front 8 Determine the Lewis Structure for acetone (CH3COCH3), Acetic Acid (CH3COOH) and isopropanol ((CH3)2CHOH) | back 8 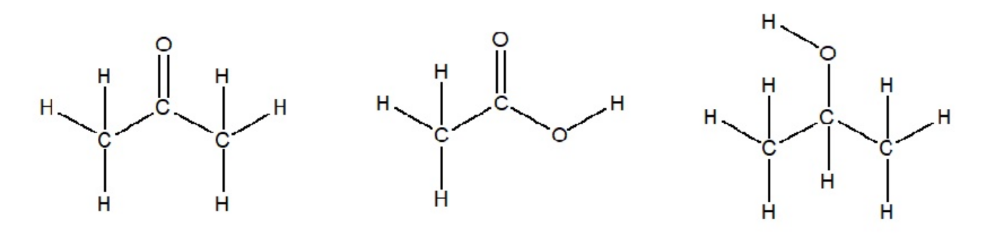 |
front 9 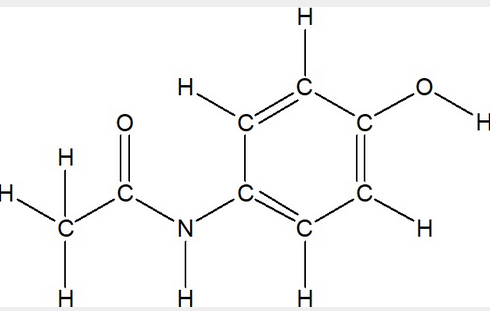 Convert the given Lewis Structure of Acetaminophen (Tylenol) into a Condensed Structure | back 9 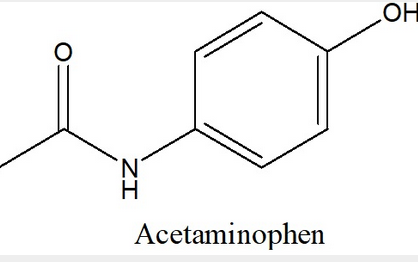 |
front 10 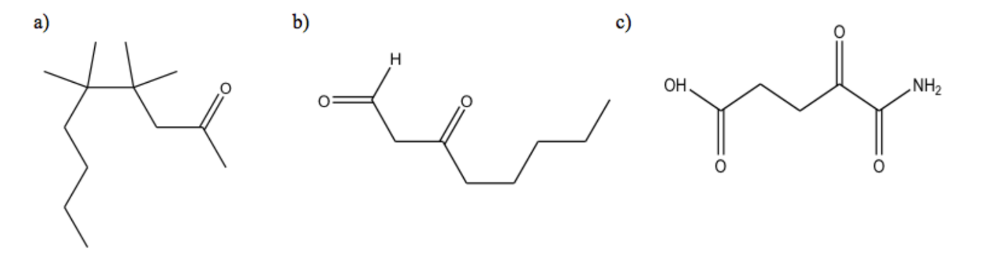 For the following compounds, give the chemical formula of the condensed structure | back 10 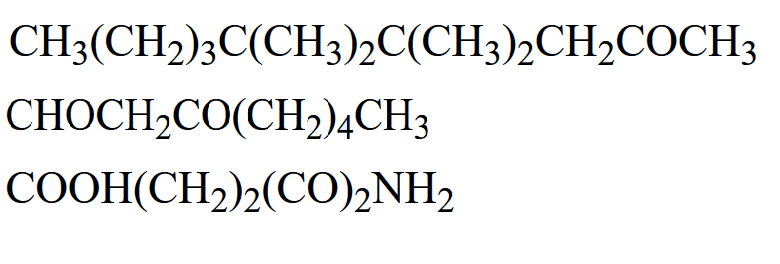 |
front 11 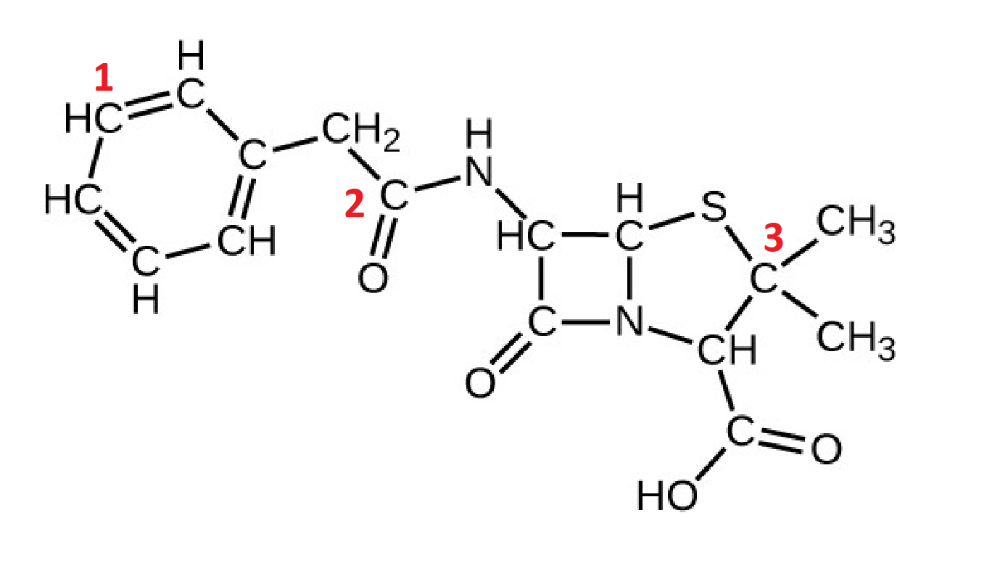 Identify the orbitals involved in the labeled carbons: C1 and H C2 and O C3 and S | back 11 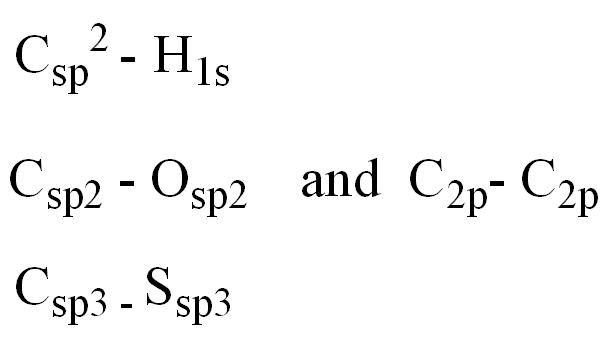 |
front 12  Determine the orbitals for the numbered atoms and bonds: C1 and adjacent Carbon N2 and H C3 and adjacent Carbon C and S4 | back 12 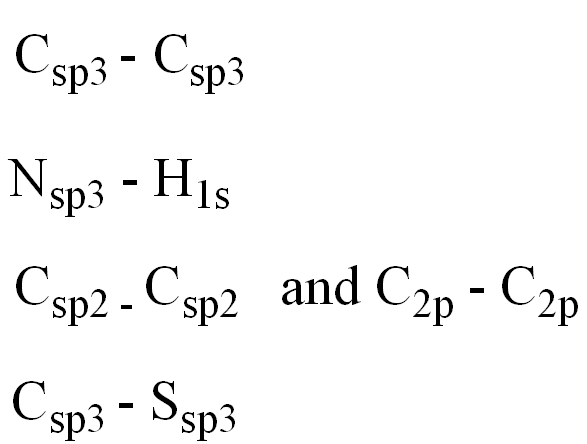 |
front 13  What is the hybridization and molecular geometry of each labeled atom? | back 13 1- sp3 hybridized and tetrahedral 2- sp2 hybridized and trigonal planar 3- sp2 hybridized and trigonal planar |
front 14 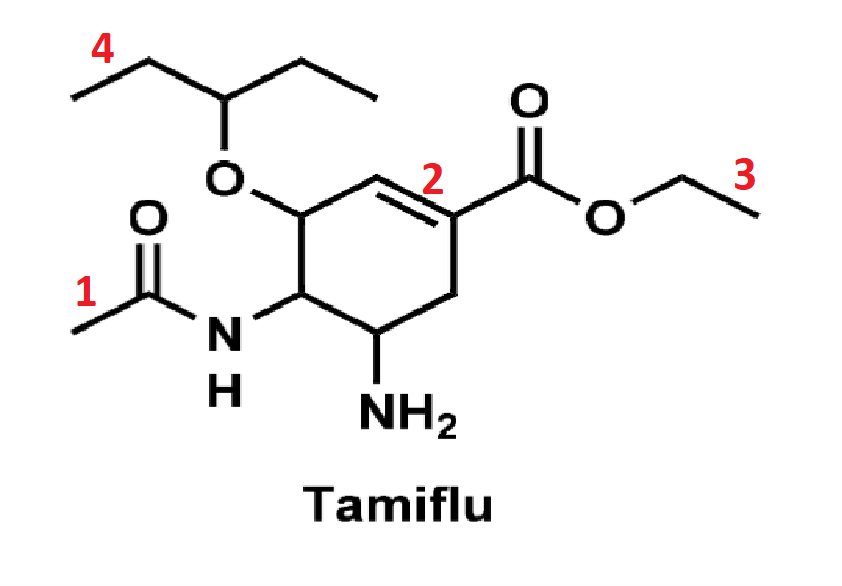 Rank the following bonds in order of increasing strength | back 14 4 < 3 < 1 < 2 |