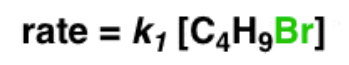Chapter 6 - Understanding Organic Reactions
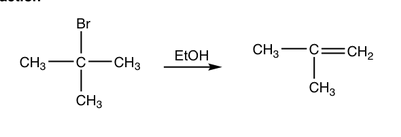
Determine whether the reaction is a substitution, elimination or addition?
Elimination
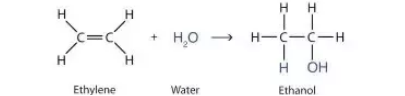
Determine whether the reaction is a substitution, elimination or addition?
Addition
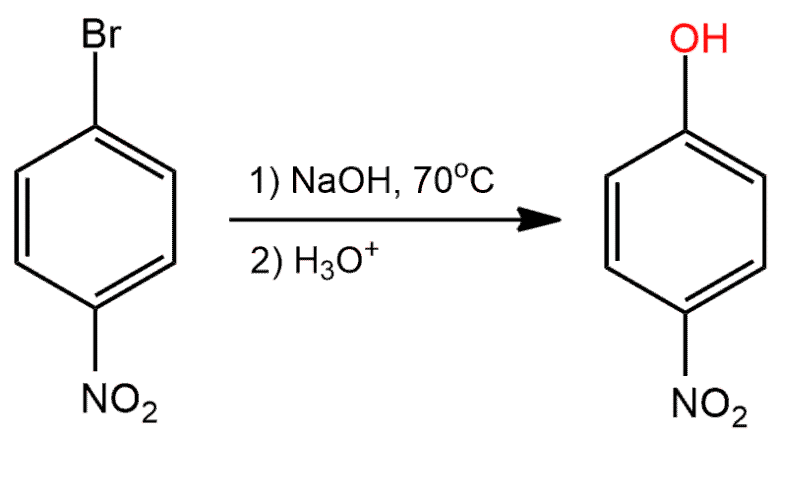
Determine whether the reaction is a substitution, elimination or addition?
Substitution
What is the difference between a radical, carbocation, and carbanion?
Radicals are formed through homolysis whereas carbocations and carbanions are formed via heterolysis. Radicals and carbocations are electrophiles whereas carbanions are nucleophiles.
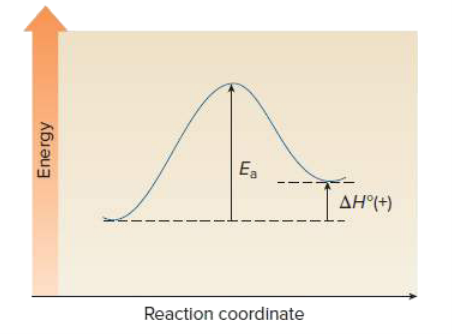
Draw an energy diagram for a one step endothermic reaction. Label key components: Axis, Energy of Activation, and Overall Enthalpy
Draw an energy diagram for a one step exothermic reaction. Label key components: Axis, Energy of Activation, and Overall Enthalpy
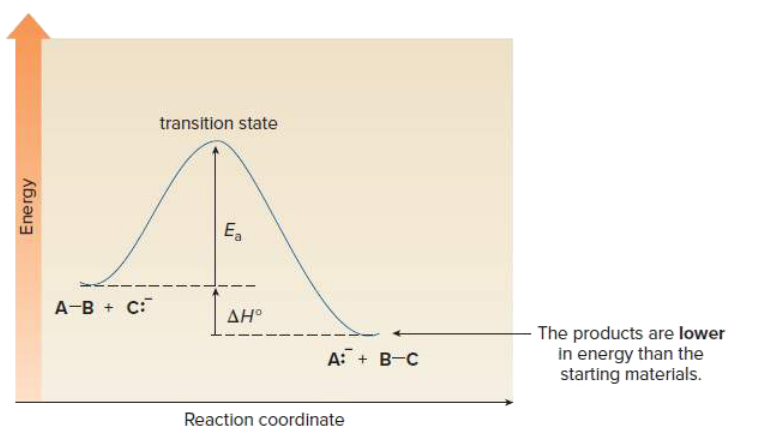
What is the difference between thermodynamics and kinetics?
Thermodynamics has to do with difference in energy between products and reactants and equilibrium, whereas kinetic describes reaction rates.
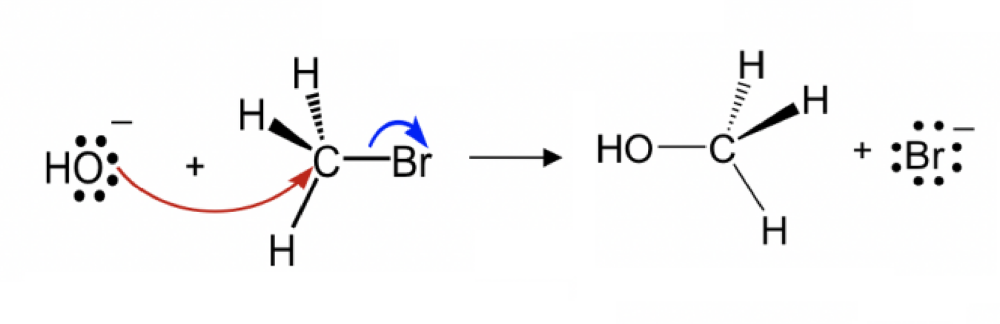
Draw the structure for the transition state in the reaction
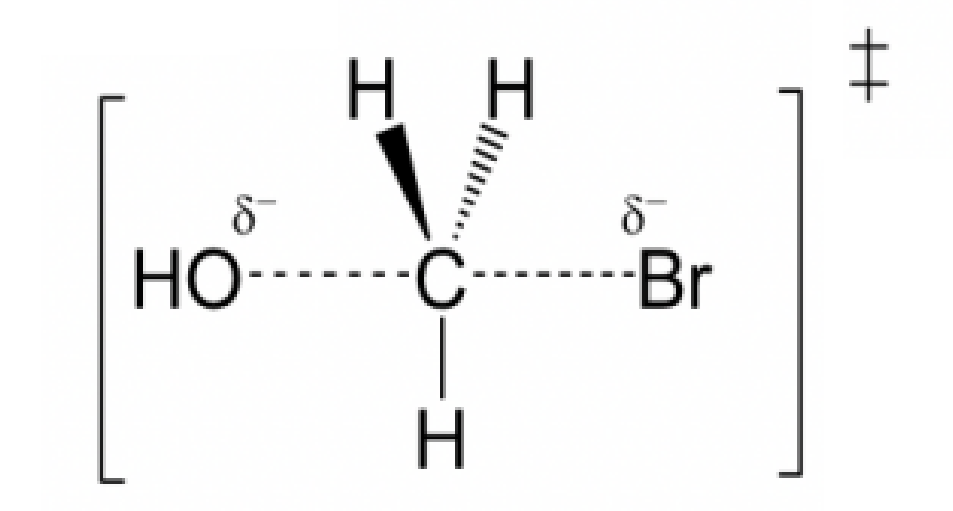
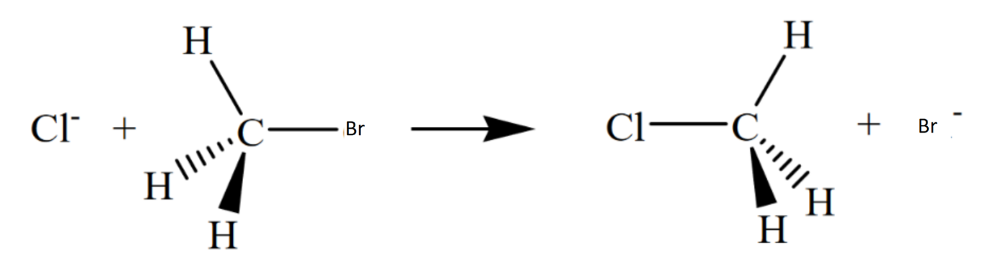
Draw the structure for the transition state in the reaction
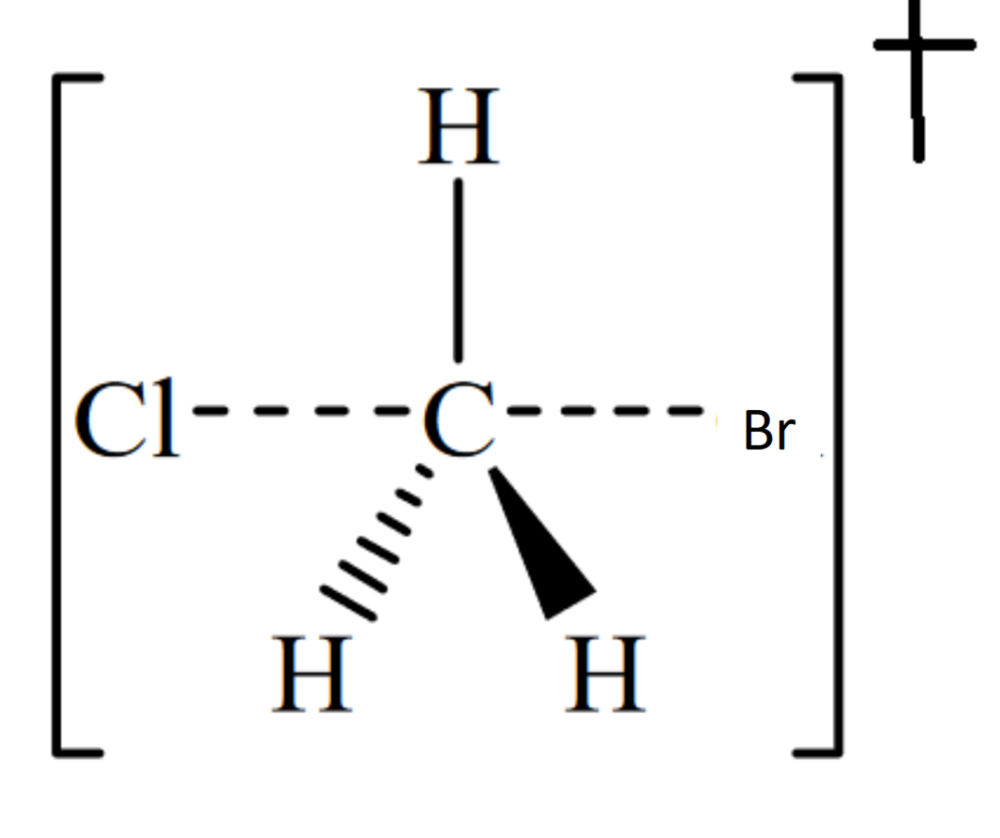
What factors affect the rate of the reaction?
Energy of activation, the concentration, and temperature. Catalysts also affect the rate.
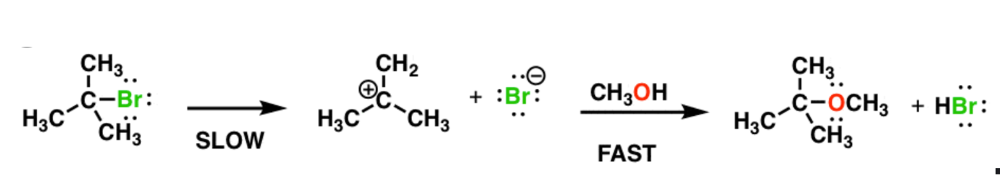
Write the rate equation for the reaction, given the indicated mechanism