Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Chapter 6 - Understanding Organic Reactions
front 1 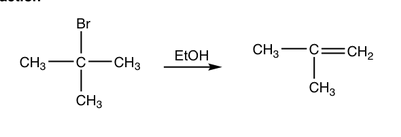 Determine whether the reaction is a substitution, elimination or addition? | back 1 Elimination |
front 2 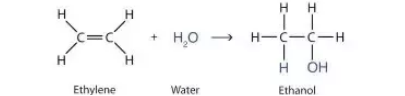 Determine whether the reaction is a substitution, elimination or addition? | back 2 Addition |
front 3 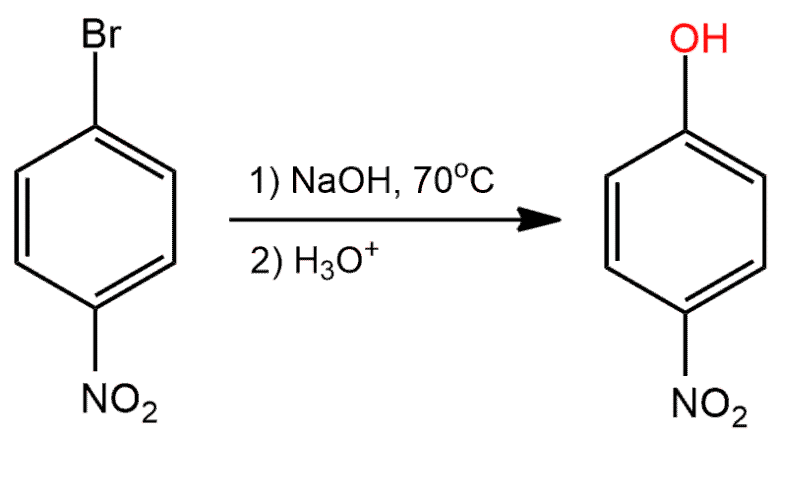 Determine whether the reaction is a substitution, elimination or addition? | back 3 Substitution |
front 4 What is the difference between a radical, carbocation, and carbanion? | back 4 Radicals are formed through homolysis whereas carbocations and carbanions are formed via heterolysis. Radicals and carbocations are electrophiles whereas carbanions are nucleophiles. |
front 5 Draw an energy diagram for a one step endothermic reaction. Label key components: Axis, Energy of Activation, and Overall Enthalpy | back 5 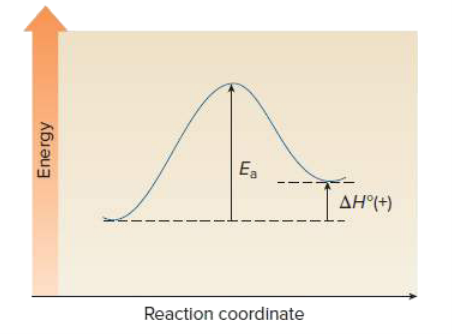 |
front 6 Draw an energy diagram for a one step exothermic reaction. Label key components: Axis, Energy of Activation, and Overall Enthalpy | back 6 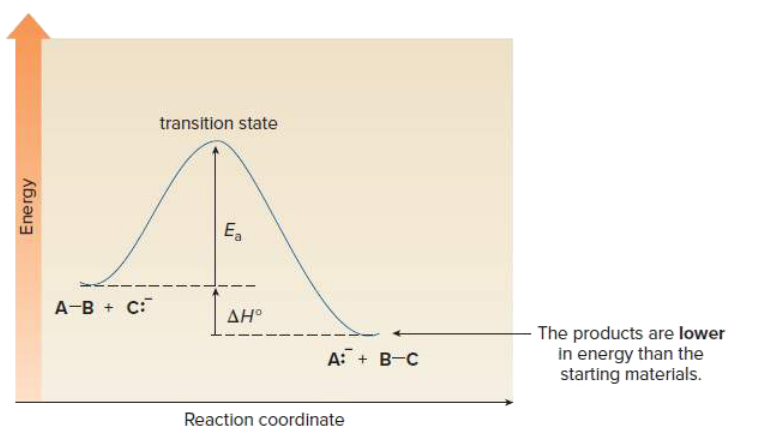 |
front 7 What is the difference between thermodynamics and kinetics? | back 7 Thermodynamics has to do with difference in energy between products and reactants and equilibrium, whereas kinetic describes reaction rates. |
front 8 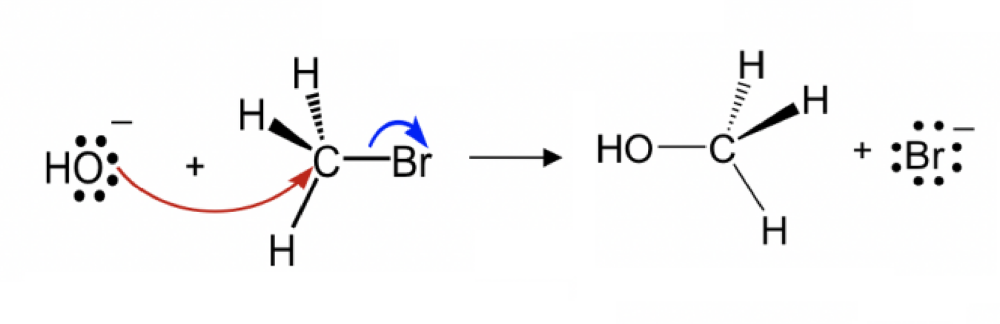 Draw the structure for the transition state in the reaction | back 8 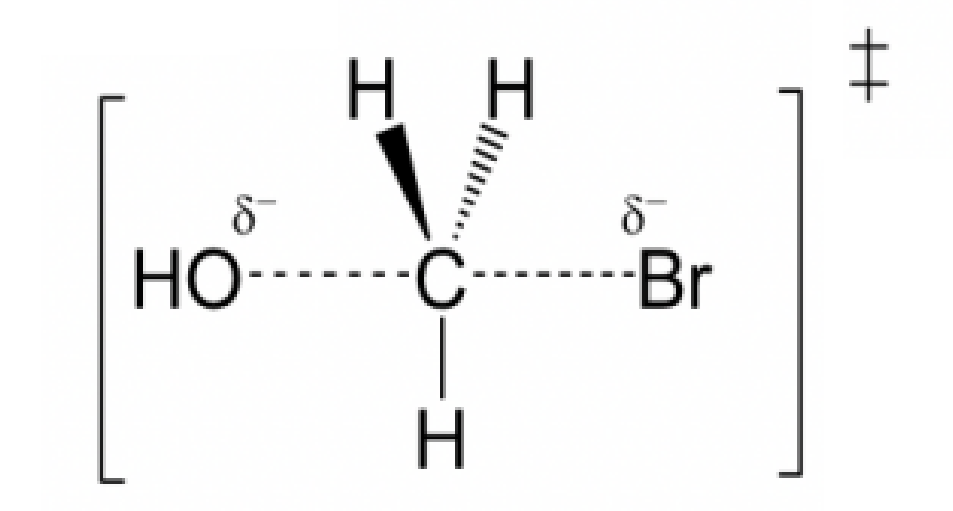 |
front 9 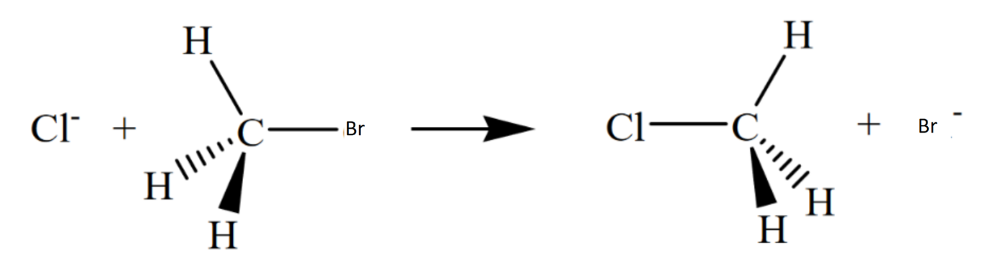 Draw the structure for the transition state in the reaction | back 9 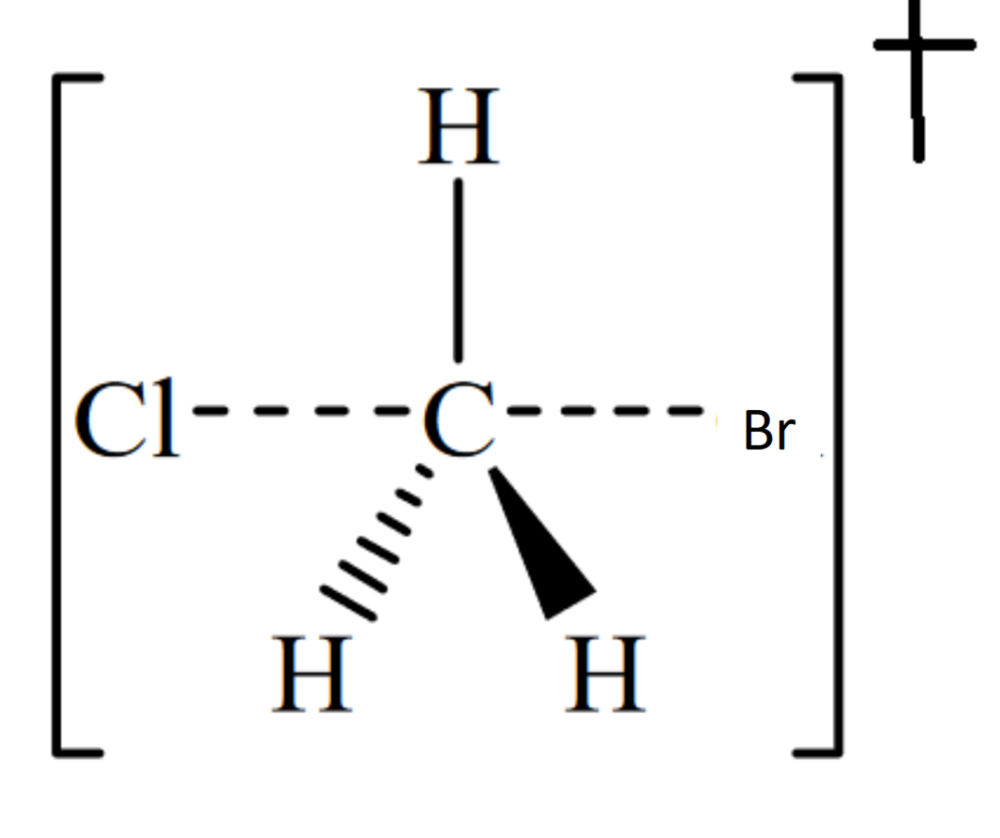 |
front 10 What factors affect the rate of the reaction? | back 10 Energy of activation, the concentration, and temperature. Catalysts also affect the rate. |
front 11 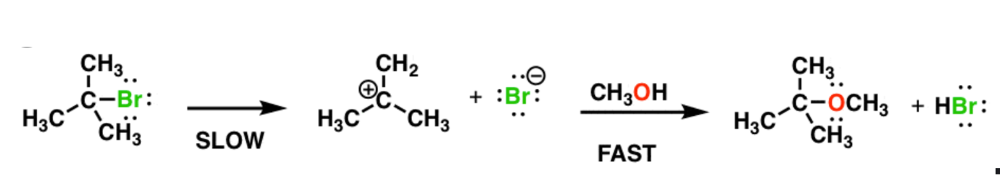 Write the rate equation for the reaction, given the indicated mechanism | back 11 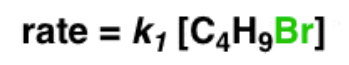 |