Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Experiment 4 Prelaboratory Assignment: Inorganic Compounds and Metathesis Reactions
front 1 1. a. Copper(II) sulfate dissolves in water to form a blue solution. What species are present in solution, i.e., what is actually swimming around in solution? Write the formulas. | back 1 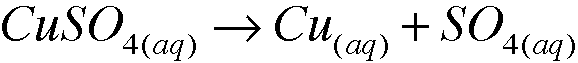 species in the solution are C2+ and SO4^2- |
front 2 1. b. Sodium carbonate carbonate dissolves in water to form a colorless solution. What species are present in solution? Write the formulas. | back 2 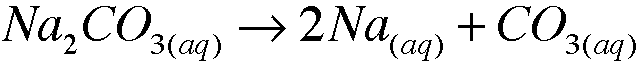 species present are Na+ and CO3^2- |
front 3 1. c. When solutions of copper(II) sulfate and sodium carbonate are mixed, blue copper(II) carbonate precipitates. What species remain in solution? (These are spectator ions.) What is the color? [Assume that stoichiometric amounts of copper(II) sulfate and sodium carbonate are mixed.] Write the formulas | back 3 Na+, CO3^2-, colorless
|
front 4 2. a. An aqueous solution of sodium hydroxide is labeled 0.010M NaOH. What are the molar concentrations of NaOH, Na+ and OH- in the aqueous solution? Explain. | back 4 0.010M NaOH, and an OH would remain because there are one mol of each |
front 5 2. b. An aqueous solution of calcium chloride is labeled 0.010 M CaCl2. What are the molar concentrations CaCl2, Ca2+, and Cl- in the aqueous solution? Explain. | back 5 CaCl2 0.010M CaCl2 –> Ca + 2Cl
|
front 6 3. Sodium phosphate dodecahydrate, Na3PO4(12H2O), is crystalline salt that is water soluble. Describe what is present in an aqueous solution of sodium phosphate dodecahydrate. | back 6 Na+ and P ions are in solution |
front 7 4. When aqueous solutions of ferrous sulfate and barium chloride are mixed, a white precipitate forms. With time the aqueous solution turns a red-orange color.
| back 7 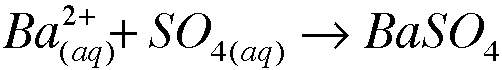 Barium sulfate |
front 8 5. Write molecular equations for each of the following metathesis reactions:
| back 8 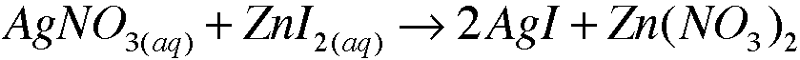 |
front 9 5. Write molecular equations for each of the following metathesis reactions:
| back 9 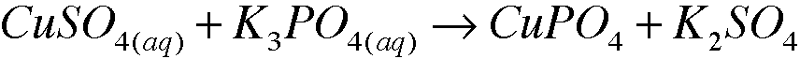 |
front 10 5. Write molecular equations for each of the following metathesis reactions: | back 10 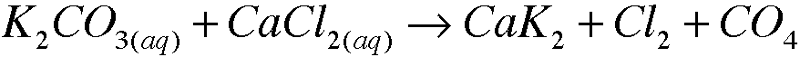 |
front 11 5. Write molecular equations for each of the following metathesis reactions:
| back 11 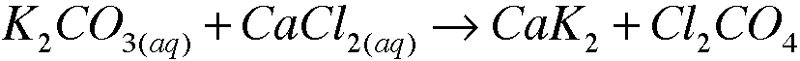 |
front 12 5. Write molecular equations for each of the following metathesis reactions:
| back 12 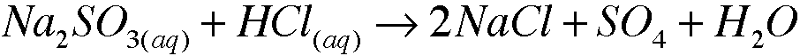 |
front 13 5. Write molecular equations for each of the following metathesis reactions:e. | back 13  |
front 14 6. List 5 observations, appealing to your senses, that indicate a chemical reaction has occurred. In each case, identify an example where you have experienced that observation. | back 14 Odor: poop
|