1) In a single molecule of water, two hydrogen atoms are bonded to a
single oxygen atom by _____.
A) hydrogen bonds
B) nonpolar
covalent bonds
C) polar covalent bonds
D) ionic bonds
Answer: C
2) The partial negative charge at one end of a water molecule is
attracted to the partial positive charge of another water molecule.
What is this attraction called?
A) a covalent bond
B) a
hydrogen bond
C) an ionic bond
D) a van der Waals interaction
Answer: B
3) The partial negative charge in a molecule of water occurs because
_____.
A) the oxygen atom donates an electron to each of the
hydrogen atoms
B) the electrons shared between the oxygen and
hydrogen atoms spend more time around the oxygen atom nucleus than
around the hydrogen atom nucleus
C) the oxygen atom has two
pairs of electrons in its valence shell that are not neutralized by
hydrogen atoms
D) one of the hydrogen atoms donates an electron
to the oxygen atom
Answer: B
4) Sulfur is in the same column of the periodic table as oxygen, but
has electronegativity similar to carbon. Compared to water molecules,
molecules of H2S will _____.
A) have greater cohesion to other
molecules of H2S
B) have a greater tendency to form hydrogen
bonds with each other
C) have a higher capacity to absorb heat for the same change in temperature D) not form hydrogen bonds with each other
Answer: D
5) Water molecules can form hydrogen bonds with _____.
A) compounds that have polar covalent bonds
B) oils
C)
oxygen gas (O2) molecules
D) chloride ions
Answer: A
6) Which of the following is a property of liquid water? Liquid water _____.
A) is less dense than ice
B) has a specific heat that is
lower than that for most other substances
C) has a heat of
vaporization that is higher than that for most other substances D) is nonpolar
Answer: C
7) Which of the following can be attributed to water's high specific heat?
A) Oil and water do not mix well.
B) A lake heats up more
slowly than the air around it.
C) Ice floats on water.
D) Sugar dissolves in hot tea faster than in iced tea.
Answer: B
8) The cities of Portland, Oregon, and Minneapolis, Minnesota, are at
about the same latitude, but Minneapolis has much hotter summers and
much colder winters than Portland. Why?
A) They are not at the
same exact latitude.
B) The ocean near Portland moderates the temperature.
C) Fresh water is more likely to freeze than salt water.
D)
Minneapolis is much windier, due to its location in the middle of
North America.
Answer: B
9) To act as an effective coolant in a car's radiator, a substance
has to have the capacity to absorb a great deal of heat. You have a
reference book with tables listing the physical properties of many
liquids. In choosing a coolant for your car, which table would you
check first?
A) pH
B) density at room temperature C) heat of vaporization
D)
specific heat
Answer: D
10) Water has many exceptional and useful properties. Which is the
rarest property among compounds?
A) Water is a solvent.
B)
Solid water is less dense than liquid water.
C) Water has a high heat capacity.
D) Water has surface tension.
Answer: B
11) Which of the following effects can occur because of the high
surface tension of water? A) Lakes cannot freeze solid in winter,
despite low temperatures.
B) A raft spider can walk across the
surface of a small pond.
C) Organisms can resist temperature
changes, although they give off heat due to chemical reactions.
D) Sweat can evaporate from the skin, helping to keep people from overheating.
Answer: B
12) Which of the following takes place as an ice cube cools a
drink?
A) Molecular collisions in the drink increase.
B)
Kinetic energy in the liquid water decreases.
C) A calorie of
heat energy is transferred from the ice to the water of the drink. D)
The specific heat of the water in the drink decreases.
Answer: B
13) A dietary Calorie equals 1 kilocalorie. Which of the following
statements correctly defines 1 kilocalorie? One kilocalorie equals
_____.
A) 1000 calories, or the amount of heat required to raise
the temperature of 1 g of water by 1°C
B) 10,000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°F
C) 1000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°C
D) 1000 calories, or the amount of heat required to raise the temperature of 100 g of water by 100°C
Answer: C
14) Which type of bond must be broken for water to vaporize?
A) ionic bonds
B) polar covalent bonds
C) hydrogen bonds
D) both polar covalent bonds and hydrogen bonds
Answer: C
15) Why does ice float in liquid water?
A) The high surface
tension of liquid water keeps the ice on top.
B) The ionic bonds
between the molecules in ice prevent the ice from sinking.
C)
Stable hydrogen bonds keep water molecules of ice farther apart than
water molecules of liquid water.
D) The crystalline lattice of
ice causes it to be denser than liquid water.
Answer: C
16) Hydrophobic substances such as vegetable oil are _____.
A)
nonpolar substances that repel water molecules
B) nonpolar
substances that have an attraction for water molecules C) polar
substances that repel water molecules
D) polar substances that
have an affinity for water
Answer: A
17) One mole (mol) of glucose (molecular mass = 180 daltons) is _____.
A) 180 × 1023 molecules of glucose
B) 1 kilogram of glucose
dissolved in 1 liter of solution
C) 180 kilograms of glucose
D) 180 grams of glucose
Answer: D
18) When an ionic compound such as sodium chloride (NaCl) is placed in water, the component atoms of the NaCl crystal dissociate into individual sodium ions (Na+) and chloride ions (Cl-). In contrast, the atoms of covalently bonded molecules (e.g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. Which of the following solutions would be expected to contain the greatest number of solute particles (molecules or ions)?
A) 1 liter of 0.5 M NaCl
B) 1 liter of 1.0 M NaCl
C) 1
liter of 1.0 M glucose
D) 1 liter of 1.0 M NaCl and 1 liter of
1.0 M glucose will contain equal numbers of solute particles.
Answer: B
19) The molar mass of glucose is 180 grams per mole (g/mol). Which of
the following procedures should you carry out to make a 1 M solution
of glucose? Into 0.8 liter (L) of water, dissolve _____.
A) 1 g
of glucose and then add more water until the total volume of the
solution is 1 L
B) 18 g of glucose and then add more water until
the total volume of the solution is 1 L
C) 180 g of glucose and
then add 0.2 L more of water
D) 180 g of glucose and then add
more water until the total volume of the solution is 1 L
Answer: D
20) You have a freshly prepared 0.1 M glucose solution. Each liter of this solution contains how many glucose molecules?
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 6.02 × 1022
Answer: D
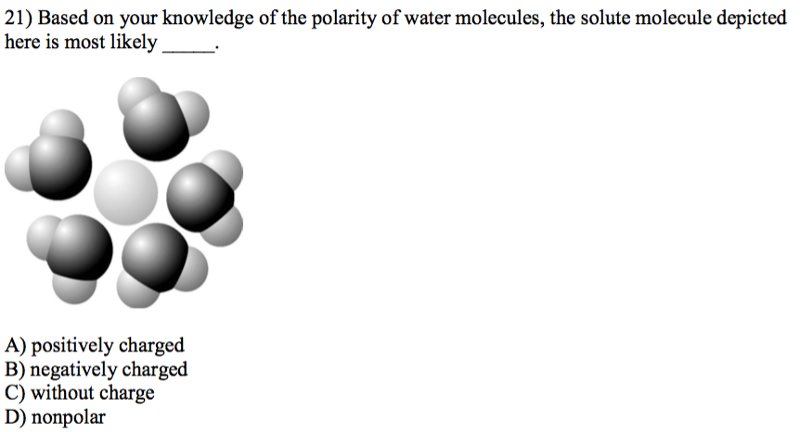
Answer: A
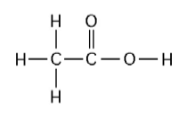
22) One mole of the compound above would weigh how many grams? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for oxygen.)
A) 29
B) 30
C) 60
D) 150
Answer: C
23) How many grams of the compound in the figure above are required
to make 1 liter of a 0.5 M solution? (Note: The atomic masses, in
daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for
oxygen.)
A) 29
B) 30
C) 60
D) 150
Answer: B
25) You have two beakers. One contains pure water, the other contains pure methanol (wood alcohol). The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. You pour crystals of table salt (NaCl) into each beaker. Predict what will happen.
A) Equal amounts of NaCl crystals will dissolve in both water and
methanol.
B) NaCl crystals will not dissolve in either water or
methanol.
C) NaCl crystals will dissolve readily in water but
will not dissolve in methanol.
D) NaCl crystals will dissolve readily in methanol but will not dissolve in water.
Answer: C
26) Rank, from low to high, the pH of blood, stomach acid, and urine.
A) blood, urine, and stomach acid
B) stomach acid, blood, and
urine
C) urine, blood, stomach acid
D) stomach acid, urine, blood
Answer: D
27) A solution with a pH of 5 has how many more protons in it than a solution with a pH of 7?
A) 5 times
B) 10 times
C) 100 times
D) 1000 times
Answer: C
28) Consider the following reaction at equilibrium: What would be the effect of adding additional H2CO3?
A) It would drive the equilibrium dynamics to the right.
B)
It would drive the equilibrium dynamics to the left.
C) Nothing
would happen, because the reactants and products are in equilibrium.
D) The amounts of CO2 and H2O would decrease.
Answer: B
29) A strong acid like HCl _____.
A) dissociates completely in
an aqueous solution
B) increases the pH when added to an aqueous solution
C) reacts with strong bases to create a buffered solution
D) is a strong buffer at low pH
Answer: A
30) Which of the following dissociates completely in solution and is
considered to be a strong base (alkali)?
A) HCl
B) NH3
C) H2CO3
D) NaOH
Answer: D
31) A 0.01 M solution of a substance has a pH of 2. What can you
conclude about this substance?
A) It is a strong acid that
dissociates completely in water.
B) It is a strong base that
dissociates completely in water.
C) It is a weak acid.
D) It is a weak base.
Answer: A
32) A solution contains 0.0000001 (10-7) moles of hydroxyl ions [OH-] per liter. Which of the following best describes this solution?
A) acidic: H+ acceptor
B) basic: H+ acceptor
C) acidic: H+ donor
D) neutral
Answer: D
33) What is the pH of a solution with a hydroxyl ion (OH-)
concentration of 10-12 M? A) pH 2
B) pH 4
C) pH 10
D) pH 12
Answer: A
34) Which of the following solutions would require the addition of
the greatest amount of base to bring the solution to neutral pH?
A) gastric juice at pH 2
B) vinegar at pH 3
C) black coffee at pH 5
D) household bleach at pH 12
Answer: A
35) What is the hydrogen ion (H+) concentration of a solution of pH 8?
A) 8 M
B) 8 x 10-6 M
C) 10-8 M
D) 10-6 M
Answer: C
36) If the pH of a solution is decreased from 9 to 8, it means that
the concentration of _____. A) H+ has decreased to one-tenth (1/10)
what it was at pH 9
B) H+ has doubled compared to what it was at
pH 9
C) H+ has increased tenfold (10X) compared to what it was
at pH 9
D) OH- has increased tenfold (10X) compared to what it was at pH 9
Answer: C
37) One liter of a solution of pH 2 has how many more hydrogen ions
(H+) than 1 liter of a solution of pH 6?
A) 4 times more
B) 40,000 times more
C) 10,000 times more
D) 100,000 times more
Answer: C
38) Which of the following statements is true about buffer
solutions?
A) They maintain a constant pH when bases are added
to them but not when acids are added to them.
B) They maintain a
constant pH when acids are added to them but not when bases are added
to them.
C) They fluctuate in pH when either acids or bases are
added to them.
D) They maintain a relatively constant pH when
either acids or bases are added to them.
Answer: D
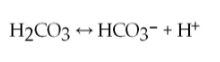
39) One of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3). Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3-) and a hydrogen ion (H+), as noted above.
If the pH of blood drops, one would expect _____.
A) a
decrease in the concentration of H2CO3 and an increase in the
concentration of HCO3- B) the concentration of bicarbonate ions
(HCO3-) to increase
C) the HCO3- to act as a base and remove
excess H+ by the formation of H2CO3
D) the HCO3- to act as an acid and remove excess H+ by the formation of H2CO3
Answer: C
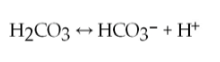
40) One of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3). Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3-) and a hydrogen ion (H+), as noted below.
If the pH of blood increases, one would expect _____.
A) a
decrease in the concentration of H2CO3 and an increase in the
concentration of HCO3- B) an increase in the concentration of H2CO3
and a decrease in the concentration of HCO3- C) a decrease in the
concentration of HCO3- and an increase in the concentration of H+
D) an increase in the concentration of HCO3- and a decrease in the concentration of OH-
Answer: A
41) Assume that acid rain has lowered the pH of a particular lake to pH 4.0. What is the hydroxide ion concentration of this lake?
A) 1 × 10-10 mol of hydroxide ions per liter of lake water
B) 1 × 10-4 mol of hydroxide ions per liter of lake water
C) 4.0 M with regard to hydroxide ion concentration
D) 4.0 × 10-4 mol of hydroxide ions per liter of lake water
Answer: A
42) Consider two solutions: solution X has a pH of 4; solution Y has a pH of 7. From this information, we can reasonably conclude that _____.
A) solution Y has no free hydrogen ions (H+)
B) the
concentration of hydrogen ions in solution Y is 1000 times as great as
the concentration of hydrogen ions in solution X
C) the
concentration of hydrogen ions in solution X is 3 times as great as
the concentration of hydrogen ions in solution Y
D) the
concentration of hydrogen ions in solution X is 1000 times as great as
the concentration of hydrogen ions in solution Y
Answer: D
43) A beaker contains 100 milliliters (mL) of NaOH solution at pH =
13. A technician carefully pours into the beaker 10 mL of HCl at pH =
1. Which of the following statements correctly describes the result of
this mixing?
A) The concentration of Na+ ions will rise.
B) The pH of the beaker's contents will increase.
C) The pH of the beaker's contents will be neutral.
D) The pH of the beaker's contents will decrease.
Answer: D
44) Increased atmospheric CO2 concentrations might have what effect on seawater?
A) Seawater will become more alkaline, and carbonate concentrations
will decrease.
B) There will be no change in the pH of seawater,
because carbonate will turn to bicarbonate.
C) Seawater will become more acidic, and carbonate concentrations
will decrease.
D) Seawater will become more acidic, and
carbonate concentrations will increase.
Answer: C
45) How would acidification of seawater affect marine organisms?
Acidification of seawater would _____.
A) increase dissolved
carbonate concentrations and promote faster growth of corals and
shell- building animals
B) decrease dissolved carbonate concentrations and promote faster
growth of corals and shell- building animals
C) increase
dissolved carbonate concentrations and hinder growth of corals and
shell-building animals
D) decrease dissolved carbonate concentrations and hinder growth of corals and shell-building animals
Answer: D
46) One idea to mitigate the effects of burning fossil fuels on atmospheric CO2 concentrations is to pipe liquid CO2 into the ocean at depths of 2500 feet or greater. At the high pressures at such depths, CO2 is heavier than water. What potential effects might result from implementing such a scheme?
A) increased carbonate concentrations in the deep waters
B)
increased growth of corals from a change in the carbonate–bicarbonate equilibrium
C) no effect because carbon dioxide is not soluble in water
D) increased acidity and decreased carbonate concentrations in the
deep waters
Answer: D
47) If the cytoplasm of a cell is at pH 7, and the mitochondrial matrix is at pH 8, then the
concentration of H+ ions _____.
A) is 10 times higher in the
cytoplasm than in the mitochondrial matrix
B) is 10 times higher in the mitochondrial matrix than in the cytoplasm
C) in the cytoplasm is 7/8 the concentration in the mitochondrial matrix
D) in the cytoplasm is 8/7 the concentration in the mitochondrial matrix
Answer: A
49) The loss of water from a plant by transpiration cools the leaf. Movement of water in transpiration requires both adhesion to the conducting walls and wood fibers of the plant and cohesion of the molecules to each other. A scientist wanted to increase the rate of transpiration of a crop species to extend its range into warmer climates. The scientist substituted a nonpolar solution with an atomic mass similar to that of water for hydrating the plants. What do you expect the scientist’s data will indicate from this experiment?
A) The rate of transpiration will be the same for both water and
the nonpolar substance.
B) The rate of transpiration will be
slightly lower with the nonpolar substance as the plant will not have
evolved with the nonpolar compound.
C) Transpiration rates will
fall to zero as nonpolar compounds do not have the properties
necessary for adhesion and cohesion.
D) Transpiration rates will
increase as nonpolar compounds undergo adhesion and cohesion with wood
fibers more readily than water.
Answer: C
50) In living systems molecules involved in hydrogen bonding almost
always contain either oxygen or nitrogen or both. How do you explain
this phenomenon?
A) Oxygen and nitrogen are elements found in
both nucleic acids and proteins.
B) Oxygen and nitrogen are
elements with very high attractions for their electrons.
C) Oxygen and nitrogen are elements found in fats and
carbohydrates.
D) Oxygen and nitrogen were both components of
gases that made up the early atmosphere on Earth.
Answer: B