How are Gibbs free energy, enthalpy, and entropy related? What does Gibbs free energy assess?
Enthalpy is the total energy in a molecule including the potential energy and entropy refers to the amount of disorder. Gibbs free energy is the amount of free energy and a change in this is computed by subtracting the change in enthalpy multiplied by temperature from the change in entropy.
- Entropy becomes more important in determining free energy change as temperature increases
Gibbs free energy assesses whether a chemical reaction is spontaneous.
- Δ G= Δ H-T Δ S
- If Δ G < 0 then reaction is spontaneous (exergonic)
If Δ G > 0 then reaction is NOT spontaneous (endergonic)
Explain the differences between the terms exothermic/endothermic and endergonic/exergonic.
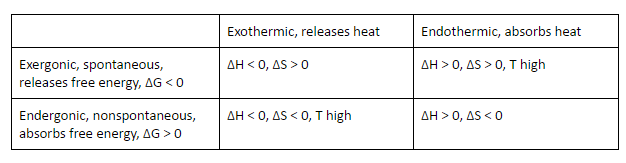
Exothermic: when a reaction releases heat energy and the products have less potential energy than the reactants, change in enthalpy is negative
Endothermic: when a reaction takes in heat energy and the products have more potential energy than the reactants, change in enthalpy is positive
Exergonic: when chemical reactions are spontaneous when change in Gibbs free energy is less than zero therefore release energy
Endergonic: when reactions are nonspontaneous when change in Gibbs free energy is greater than zero therefore require an input of energy to proceed
What conditions could you change to make a nonspontaneous reaction spontaneous? Explain, using mathematical equations.
Temperature.
If Δ H </= 0 and temperature and ΔS >/= 0 then ΔG is negative and reaction becomes spontaneous
In living cells, reactions that are energetically coupled allow energy released from one reaction to drive another reaction. Describe two mechanisms for energetic coupling of reactions in cells.
Reduction-oxidation reactions: chemical reactions that involve the loss or gain of one or more electrons, an electron can be completely transferred from one atom to another or shift position in a covalent bond, sometimes the electron can be accompanied by a proton (H+), energy lost increases the potential energy of the reduced molecule
- Oxidation: loss of electrons, exergonic half reaction
- Reduction: gain of electrons, endergonic half reaction
ATP hydrolysis: hydrolysis of ATP’s outermost phosphate group is a highly exergonic reaction and results in the formation of ADP and P, releases free energy
- Phosphorylation: the addition of a phosphate group to a substrate
- When reactant molecules in an endergonic reaction are phosphorylated, the free energy released during phosphorylation is coupled to the endergonic reaction to make the combined overall reaction exergonic.
- Activated substrates have high enough potential energy that the reaction becomes exergonic
Explain the role enzymes play in chemical reactions. Do enzymes make endergonic reactions spontaneous? If so, describe the mechanism. If not, explain how do they catalyze reactions?
Enzymes bring substrate molecules together in a substrate binding site known as the enzyme’s active site and help substrates collide in a precise orientation so that the electrons involved in the reaction can interact, many enzymes undergo a change in shape when binding occurs and this is called induced fit
- Induced fit: when an enzyme changes shape after molecule binds to active site
- Transition state: a high-energy intermediate state of the reactants during a chemical reaction that must be achieved for reaction to proceed (when “key” is in “lock”)
- Activation energy: certain amount of kinetic energy that is required to strain the chemical bonds in substrates so they can achieve transition state
No, interactions with amino acid R-groups at the enzyme active site stabilize the transition state and thus lower the activation energy required for the reaction to proceed by lowering the free energy.
- An enzyme only changes the free energy of the transition state
- The more unstable the transition state, the higher the activation energy and the less likely a reaction is to proceed quickly
What is a substrate? How does the rate of an enzyme-catalyzed reaction change as a function of substrate concentration?
A substrate is the substance on which an enzyme acts.
Substrate: molecule/reactant that interacts with a catalyst such as an enzyme or ribosome in chemical reactions
The rate of an enzyme-catalyzed reaction increases in a steep linear fashion when substrate concentrations are low and the speed begins to slow at intermediate concentrations. The reaction rate plateaus at a maximum speed at high substrate concentration.
In order for a substrate and enzyme to create a reaction, there must be enough kinetic energy to overcome repulsion between 2 electrons that have come into contact as a bond forms, and both substrate and enzyme must collide in a precise orientation.
What factors affect enzyme function? Specify the effect each factor has at the molecular level.
Ph affects the charge on carboxyl and amino groups in residue side chains and also the active sites ability to participate in reactions that involve the transfer of protons or electrons
Temperature affects the folding and movement of an enzyme as well as the kinetic energy of its substrates
- When temperature goes up, reaction speed goes up
If concentration of substrates increases, reaction rate increases
Cofactors (inorganic ions which reversibly interact w/ enzymes), coenzymes (organic molecules that reversibly interact . enzymes ex. NADH, FADH2), and prosthetic groups (non-amino acid atoms or molecules that are permanently attached to proteins) all help enzymes
How are enzymes regulated? Describe at least two types of noncovalent modifications and one type of covalent modification. What effect does enzyme regulation have on the rate of an enzyme-catalyzed reaction?
Enzymes are regulated by other molecules (sometimes other enzymes). They regulate by changing the structure of the enzyme in a way that their activity activates/inactivates the enzyme. All regulation strategies depend on the concentration of regulatory molecules.
Noncovalent modification: molecules that regulate enzyme activity by binding noncovalently to the enzyme to either activate it or inactivate it, this process is reversible because it doesn’t alter the enzyme’s primary structure
- Competitive inhibition: the regulatory molecule is similar in size and shape to the enzyme's natural substrate and inhibits catalysis by binding to the enzyme’s active site
- Allosteric regulation: the regulatory molecule binds at a location other than the active site and changes the shape of the enzyme making the active site available or unavailable
Covalent modifications: the function of an enzyme is altered by a chemical change in its primary structure, this change may be reversible or irreversible.
- Irreversible changes often result from the cleavage of peptide bonds that make up the primary structure of the enzyme
- The addition of one or more phosphate groups to the enzyme adds a negative charge to one or more amino acid residues in a protein, the electrons in that part of the protein change configuration and the enzyme’s conformation changes as well
Explain how feedback inhibition can regulate a metabolic pathway. What would happen if feedback inhibition were removed?
Feedback inhibition occurs when an enzyme in a pathway is inhibited by the product of the reaction sequence
This is a convenient way for pathways to shut themselves down when their activity is no longer needed because as the concentration of the product molecule becomes abundant, it “feeds back” to stop the reaction sequence.
Early inhibition allows initial quantity of substrate to get stored for later
If it were removed, amount of substrate would decrease quickly and there would not be enough substrate for future reactions