For the simple reaction Y → X, the equilibrium constant K is:
[x]/[y]
The equilibrium constant is a ratio of product to substrate where [X] is the concentration of product at equilibrium and [Y] is the concentration of substrate.
The free-energy change (ΔG) and the standard free-energy change (ΔG°) of a reaction differ in that ΔG depends on the concentrations of the molecules in the reaction, whereas for ΔG°, these concentrations are set to a fixed value.
True
The standard free-energy change, ΔG°, depends only on the intrinsic characters of the reacting molecules.
For a reaction at 37°C, a 1.42 kcal/mole decrease in ΔG, changes K by a factor of...
10
As the free-energy change for a reaction becomes more negative, the reaction becomes more energetically favorable. The more energetically favorable the reaction, the more product will accumulate, if the reaction proceeds to equilibrium.
Consider the reaction A + B → AB. How is the equilibrium constant expressed for this reaction with two substrates and a single product?
K = [AB] / [A][B]
In a reaction with two substrates, the equilibrium constant must take into account the concentrations of both reactants, in addition to that of the product; the concentrations of the reactants are multiplied because the collision of A and B to form product is proportional to [A] x [B].
Two molecules will bind to each other by means of noncovalent bonds if the ΔG° of the interaction is:
negative (the free energy of the product is lower than the sum of the free energies of the unbound partners).
Two molecules will bind to each other if the free energy of their interaction is negative.
Consider two molecules that associate with each other through hydrogen bonds. The larger the equilibrium constant, K, for this association:
the more tightly the two molecules will bind.
By convention, the equilibrium constant is expressed as a ratio of product to substrates. Thus, the equilibrium constant becomes larger as the energy released in the binding interaction increases.
The overall free-energy change for coupled reactions is equal to the sum of the free-energy changes for each individual reaction.
True
If the sum of the free-energy changes of each reaction is negative, the coupled reaction will be energetically favorable.
In cells, small molecules can diffuse over short distances quickly.
True
Because of heat energy, small molecules are in constant motion and can move rapidly across short distances.
In a cell, the rate at which an enzyme will encounter its substrate depends on:
the concentration of the substrate.
The most abundant substrates, present at a concentration of 0.5 mM, will collide with their enzymes approximately 500,000 times per second.
In an enzyme-catalyzed reaction, a small value of K M indicates that a substrate binds:
very tightly to the enzyme.
The K M is the concentration of substrate at which the enzyme works at half its maximum speed; the smaller the K M, the more tightly the substrate binds.
When an enzyme lowers the activation energy for the forward reaction X → Y, it also lowers the reaction rate for the reverse reaction Y → X by the same amount
True
The equilibrium point for the reaction thus remains unchanged.
In the second step of glycolysis, the pathway that begins the oxidative breakdown of sugars, the enzyme phosphoglucose isomerase converts glucose 6-phosphate to fructose 6-phosphate. The equilibrium constant, K, for this reaction is 0.36. If ΔG° = –1.42 x log K, which conclusion can be made about this reaction?
The ΔG° is positive, but in a cell that is actively burning sugars, the reaction can still proceed in the forward direction.
For this reaction, ΔG°is positive.
0.36 = 3.6 x
10-1, so its log is a negative number (because of the
–1).
As ΔG° = –1.42 * log K, and log
K in this case is negative, then ΔG° will be
positive. In cells actively consuming glucose, fructose 6-phosphate is
present at lower concentrations than glucose 6-phosphate, which gives
the reaction a negative ΔG. In effect, subsequent reactions
in the glycolysis pathway that are energetically favorable act as a
“siphon,” pulling the previous reactions in the forward direction.
At equilibrium, the free energy of a reaction is:
At its lowest Point.
Free energy is the energy that can be harnessed to do work. At equilibrium, ΔG = 0, so the rates of the forward and reverse reactions are equal and no work can be done.
When the concentrations of substrate and product are equal:
ΔG = ΔG°.
When the reactants and products are present in equal concentrations,
the direction of the reaction depends entirely on the intrinsic
properties of the molecules involved. Remember that
ΔG =
ΔG° + RT x the natural log of [product]/[reactant].
When [product]/[reactant] = 1, ΔG = ΔG° because the natural
log of 1 = 0.
When an enzyme is operating at its maximum rate:
the substrate-binding sites on the enzyme molecules are fully occupied
When a solution of enzyme is operating at V max, the active sites of all of the enzymes in the sample are occupied by substrate. At this point, the rate of product formation depends only on how rapidly the substrate molecules can undergo the chemical reaction that converts them into product.
Inhibitor molecules can block an enzyme’s activity. Some inhibitors, called competitive inhibitors, compete directly with the substrate for the enzyme’s active site. Such inhibitors will:
have no effect on the V max of the reaction
Competitive inhibitors do not change the V max of a reaction. If enough substrate is added, it essentially guarantees that the enzyme will always encounter a substrate molecule (rather than an inhibitor). And when the enzymes are fully loaded with substrate, they can operate at maximal velocity.
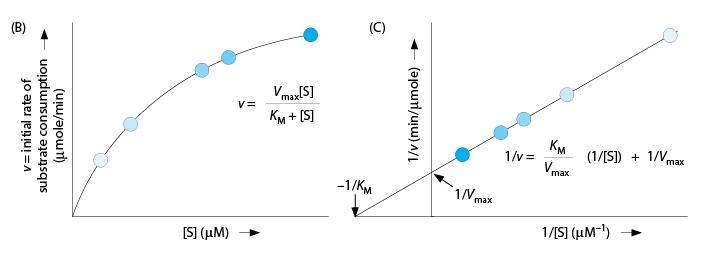
When studying an enzyme’s kinetics, it can sometimes be difficult to calculate exact values for V max and K M based solely on a curve that plots the velocity of the reaction at different concentrations of substrate. To remedy this situation, biochemists convert the data to reciprocals and generate a “double-reciprocal plot.” A standard plot and its double-reciprocal plot are shown here. The darkness of each data point (blue) represents the relative substrate concentrations.
Examining the double-reciprocal plot, which letter corresponds to a location that would allow the calculation of the enzyme’s V max?
C.
C marks the “y intercept”—the point at which the curve hits the y axis. The fact that the y axis represents the reciprocal of the velocity, 1/v, suggests you are on the right track. Further, extrapolating from the lowest to the highest substrate concentrations (lightest to darkest data points) leads you to the y intercept.
Small molecules diffuse through the cytosol very efficiently by:
wandering randomly, knocked around by collision with other molecules.
Although it doesn’t sound very efficient, small molecules in solution are bounced around by colliding with other molecules. Such interactions allow them to diffuse through the cytosol—randomly but rapidly