nutrient
Any substance which can be metabolized by an organism to provide useful chemical energy and build tissue; components of food which fall into the general categories: carbohydrates, lipids, proteins, nucleic acids, vitamins and minerals.
metabolism
The chemical processes occurring within a living cell or organism that are necessary for the maintenance of life. In metabolism some substances are broken down to yield energy for vital processes while other substances, necessary for life, are synthesized.
ATP = adenosine tri-phosphate
A nucleotide [C10H16N5O13P3] which contains the purine adenine, a pentose sugar (ribose or deoxyribose) and three high-energy phosphate groups; the potential chemical energy in the bonds connecting the phosphate groups is used to transport energy within cells for biochemical processes (including muscle contraction and enzymatic metabolism) through its hydrolysis; the mitochondrion is the primary cell organelle synthesizing this compound using energy derived from the final oxidation of nutrient molecules.
catabolism
The metabolic breakdown of complex molecules into simpler ones, resulting in a release of energy. These are the breakdown reactions of metabolism and are exergonic/exothermic; they may or may not yield an output of useful chemical energy.
anabolism
The phase of metabolism in which simple substances are synthesized into the complex materials of living tissue. These are the synthetic reactions of metabolism and are endergonic/endothermic; they require an input of useful chemical energy.
basal metabolic rate (BMR)
The rate at which energy is used by an individual at complete rest, measured by the heat given off per unit time, and expressed as the calories released per kilogram of body weight or per square meter of body surface per hour; it is regulated by thyroid hormones (T3 and T4) and epinephrine.
glucose catabolism
The series of linked enzyme catalyzed steps by which the 6-carbon monosaccharide glucose is broken down into smaller molecules with the production of some useful chemical energy for the cell; it follows two major routes: (1) anaerobic fermentation in which one glucose yields two lactate molecules and 2ATPS; this reaction, anaerobic glycolysis, occurs in the cytoplasm of cells, and (2) aerobic cellular respiration in which one glucose yields 6 carbon dioxide molecules, 12 water molecules and a maximum of 38 ATPSs; this reaction, glycolysis, begins in the cytoplasm but continues in the mitochondria (pyruvate decaroxylation, citric acid cycle, electron transport system, and oxidative phosphorylation).
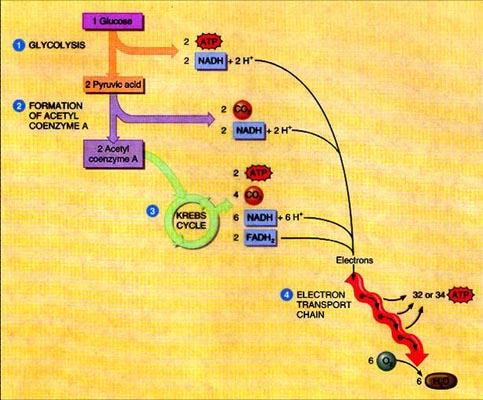
Diagram: glucose catabolism
glycogenesis
The anabolic synthesis of glycogen (animal starch) from glucose monomers using ATP energy which occurs primarily in hepatocytes and skeletal muscle cells; it is encouraged by insulin during the absorptive state.
glycolysis
The first and cytoplasmic portion of glucose catabolism in which glucose is converted in a series of linked enzyme catalyzed steps to pyruvic acid and useful chemical energy (net gain 2 ATPs under anaerobic conditions or net gain 2 ATPs and 2 NADH2's under aerobic conditions); techically, either route is considered anaerobic because no molecular O2 is required in any of the steps.
glycogenolysis
The enzymatic breakdown of glycogen to release glucose from the liver, primarily during the postabsorptive state, which is triggered by the action of various hormones, e.g., glucagon, human growth hormone, glucocorticoids, which may all be referred to as “insulin antagonists.”
gluconeogenesis
The formation of "new" glucose, especially by the liver, primarily during the postabsorptive state, from noncarbohydrate sources, such as pyruvate, amino acids and the glycerol portion of fats; this process is stimulated by those hormones termed "insulin antagonists," which includes glucagon, human growth hormone, and the glucocorticoids.
lipogenesis
The anabolic synthesis of neutral fats (triglycerides) or other lipids from a variety of precursor nutrients including other fats, carbohydrates, or proteins; it is one of the major functions of adipocytes and one of the many functions of hepatocytes.
lipolysis
The hydrolysis of lipids which may lead to fat catabolism which will result in a release of useful chemical energy; this process is stimulated by those hormones termed insulin antagonists, which includes glucagon, human growth hormone, and the glucocorticoids; it occurs primarily during the postabsorptive state.
Describe: the relationship between catabolic pathways and anabolic pathways in metabolism.
Catabolic pathways consist of reactions in which larger molecules are chemically broken apart into smaller component molecules. Catabolic pathways may require an input of energy of activation to get them started, but they are exothermic and exergonic, which means they liberate energy in one form or another as one of the products of the reaction. Some catabolic pathways yield useful chemical energy in the form of ATP or its equivalents or energetic electrons (with associated hydrogen ions) carried by specific electron transport compounds such as NAD+ and FAD+. Those catabolic pathways which liberate useful chemical energy may be coupled to anabolic pathways in order to transfer the useful chemical energy to drive the anabolic reaction. Anabolic pathways consist of reactions in which smaller component molecules are chemically joined together to make larger molecules. Anabolic pathways require considerable input of external chemical energy in order to form the bonds which link the component molecules. They are termed endothermic and endergonic for this reason.
Describe: the relationship between useful chemical energy and waste heat energy in metabolic reactions.
All chemical reactions involve the making and breaking of chemical bonds. The total amount of chemical energy in the reactants will not be the same as the total amount of chemical energy in the products. Even spontaneous reactions generally require some external source of energy of activation to initiate the reaction. All reactions are less than 100% efficient in energy usage and transfer and, therefore, all reactions lose some energy in the form of random molecular movement which is defined as "waste heat." In a subset of those reactions which can be termed catabolic, exothermic and exergonic, some of the energy is transferred to another form, other than waste heat. This energy may be mechanical energy of motion, creation of a voltage potential, or the storage of chemical energy in the chemical bonds of some compound. In some cases, that chemical energy is then able to be used in coupled reactions to drive other reactions. Examples of product molecules which retain this sort of useful chemical energy include ATP, NADH+ + H+ and FADH+.+ H+.
Diagram: Heat Balance
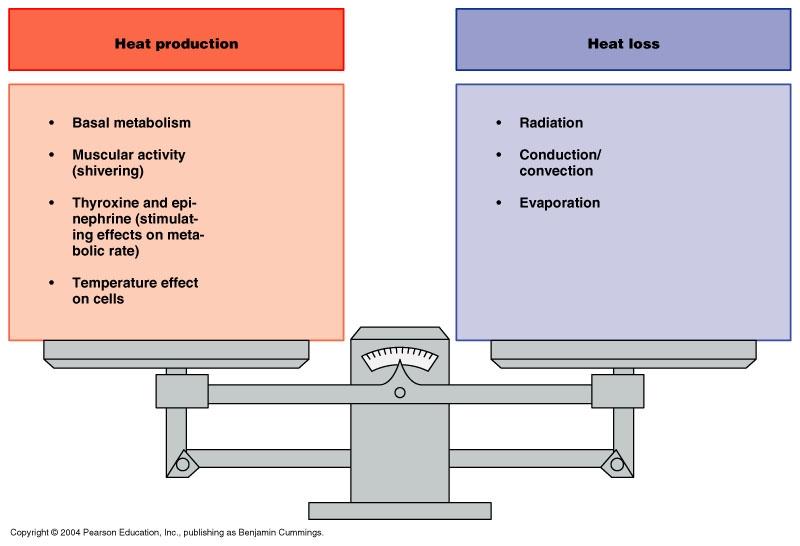
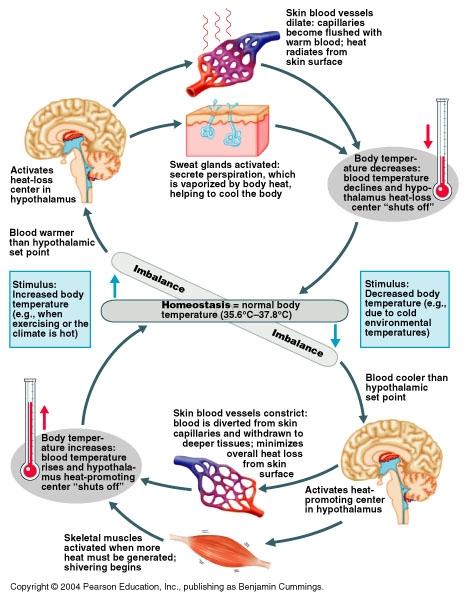
Diagram: body temperature regulation
thermoregulation
The maintenance of a constant internal body temperature independent from the environmental temperature; the skin, sweat glands, subcutaneous adipose tissue, and dermal capillary beds lay important roles in thermoregulation.
behavioral thermoregulation
Activities which are responses in changes in internal or external temperatures, such as making changes in posture, or exercising, or moving about in the environment, or drinking hot or cold beverages, or adding or removing layers of clothing, or huddling with other individuals, or seeking shelter, or using fire or other environmental thermoregulatory technologies, to control body temperature;
thermogenesis
The generation or production of heat, especially by physiological and metabolic processes such as increased basal metabolic rate (BMR); these processes are regulated by thyroid hormones (T3 and T4), and epinephrine, and by shivering which is regulated by the CNS.
hypothalamic thermostat
A center in the anterior portion of the hypothalamus of the brain, where there are neurons sensitive to temperature changes reported by sensory thermoreceptors located in the skin, mucous membranes, circulatory system and the brain itself, which regulates body temperature; it works in conjunction with other hypothalamic, autonomic and higher nervous thermoregulatory centers to keep the core temperature constant; some of these thermoregulatory responses are involuntary, mediated by the autonomic nervous system, some are neurohormonal and others are semi-voluntary or voluntary behavioral responses.
thyroid hormones
(1) amines: thyroxine = tetraiodothyronine = T4 and triiodothyronine = T3 which are produced by follicular cells in response to TSH and target most cells in the body to increase their cellular metabolism, a thermogenic effect; and (2) protein: (thyro)calcitonin produced by the autoregulated parafollicular = C cells released in response to increases in plasma calcium ion levels which targets osteoblasts to increase formation of bone matrix causing a corresponding decrease in plasma calcium and phosphate ion levels.
vasoconstriction `
Any decrease in the diameter of blood vessels due to the contraction of the smooth muscle in the vessel's walls; usually regulated by the autonomic NS and certain hormones.
chemical thermogenesis
The generation or production of heat by physiological and metabolic activities which increase the basal metabolic rate (BMR); this is regulated by the hypothalamus which stimulates the release of (1) TSH from the anterior pituitary in order to increase output of T3 and T4 from the thyroid gland and the release of (2) epinephrine from the adrenal medulla.
shivering thermogenesis
generation or production of heat by stimulating involuntary uncoordinated contractions of the skeletal muscles which is regulated by a shivering center in the hypothalamus which then activates motor centers in the brain stem; these contractions require catabolism of nutrients for energy production which provide most of the heat production.
cutaneous vasodilation
A increase in the diameter of the superficial blood vessels supplying the capillary beds of the dermis of the skin due to the contraction of the smooth muscle in the vessels' walls; usually regulated by the autonomic NS and certain hormones; it increases blood flow in the skin and typically increases heat transfer to a cool environment.
cutaneous vasoconstriction
A decrease in the diameter of the superficial blood vessels supplying the capillary beds of the dermis of the skin due to the contraction of the smooth muscle in the vessels' walls; usually regulated by the autonomic NS and certain hormones; it reduces blood flow and heat transfer to the environment; heat loss from a human is reduced when arms and legs cool to several degrees below the temperature of the body core, where most vital organs are located.
radiation
The propagation of energy through space; the emission of energy in the form of particles or electromagnetic rays or waves; in the case of thermoregulation, the conversion of body heat into infrared rays which are transmitted away from the body.
evaporation
The process by which any substance is converted from a liquid state into, and carried off in, a vapor or gas state; this process requires an input of energy (heat) and the surface (solid or liquid) from which the vapor escapes is therefore cooled by the process
conduction
The transmission of something through a medium or passage, especially the transmission of electric charge or heat through a conducting medium without perceptible motion of the medium itself; the direct transfer of thermal motion (heat) between molecules in direct contact with each other; heat is always conducted from an object of higher temperature to one of lower temperature; however, the rate and amount of heat transfer varies with different materials; water is 50 to 100 times more effective than air in conducting heat.
convection
Heat transfer in a gas or liquid by the circulation of currents from one region to another; the transfer of heat by the movement of air or liquid within the medium or past a surface; it is caused by the molecular motion; it occurs when a breeze contributes to heat loss from the surface of an animal with dry skin; it also occurs when circulating blood moves heat from an animal’s warm body core to the cooler extremities such as legs.
fever
A rise in the temperature of the body above the normal range; frequently a symptom of infection; stimulated by various chemicals, some of which are present in microorganisms (exogenous pyrogens) while others are internal local hormones released by immune system cells (endogenous pyrogens).
pyrogen
A substance that produces fever; various chemicals with this property are known, some of which are present in microorganisms (exogenous pyrogens) while others are internal local hormones released by immune system cells (endogenous pyrogens); the major endogenous pyrogen in humans is probably interleukin-1.
chill
A sensation of coldness, often accompanied by shivering and pallor of the skin, a pinched face, and blue lips, caused by undue cooling of the body or by nervous excitement, or forming the precursor of some constitutional disturbance, e.g., a fever; often an early symptom of infectious disease due to the invasion of the body by toxins; to be seized with cold.
hypothermia
An abnormally low body temperature, due to exposure to cold weather, or immersion in cold water, or induced by medical means to decrease the metabolism of tissues and, thereby, the need for oxygen, during certain surgical procedures, especially on the heart.
heat stroke
A severe condition caused by impairment of the body's thermoregulatory abilities, resulting from prolonged exposure to excessive heat and characterized by cessation of sweating, severe headache, high fever, hot dry skin, and in serious cases collapse and coma.
hyperthermia
An abnormally high body temperature, due to high fever, over exposure to the sun and heat stroke, burns, severe viral infections, bacterial septicemia and certain toxins, thyrotoxic crisis, as an undesirable side effect of certain drugs used in general anesthesia (anesthetics and muscle relaxants), etc., or induced for therapeutic purposes.
List: the systems and control factors involved in negative feedback regulation of body temperature.
thermoreceptors located in the hypothalamus monitor internal temperature
the hypothalamus initiates autonomic commands which regulate dilation or constriction of cutaneous (dermal) capillary beds and initiation of sweating or shivering
the hypothalamus signals the anterior pituitary = adenohypophysis to release more or less Thyroid Stimulating Hormone (TSH) to alter thyroid gland hormone output and, therefore, alter basal metabolic rate (BMR)
conscious recognition of significant temperature changes may stimulate appropriate changes in behavior
List ways the body produces heat.
increasing thyroid hormone activity raising basal metabolic rate (BMR)
increasing epinephrine hormone activity raising basal metabolic rate (BMR)
shivering of skeletal muscles
changing behavior: start exercising
List ways the body conserves heat.
vasoconstriction of cutaneous (dermal) capillary beds
changing behavior: huddle together; move into a warmer environment (into the sunlight; a warm place); add layers of clothes or other forms of insulation; start a fire or turn on a heating system
List ways the body loses heat to the environment.
infrared radiation from body surfaces to the environment
conduction and convection to air or water around the body
water evaporation from sweating and from ventilating wet respiratory membranes
vasodilation of cutaneous (dermal) capillary beds
decreased thyroid hormone activity lowering basal metabolic rate (BMR)
changes in behavior: stop exercising; move into the shade; remove clothes; turn on air conditioning
essential amino acids
Those building blocks, i.e., monomers (amino acids), of proteins, which are mandatory for normal growth and development but cannot be synthesized or are synthesized too slowly for normal requirements; therefore, these essential amino acids must be obtained from the diet; the group includes: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
non-essential amino acids
Those building blocks, i.e., monomers (amino acids), of proteins, which may be synthesized within the body and, therefore, are not mandatory within the diet; they include: alanine, arginine, aspartate, glutamate, glycine, proline, and serine. [Note: As cysteine and tyrosine require the presence of methionine and phenylalanine respectively, they may be considered as non-essential amino acids only if their precursors are available within the diet.]
minerals
Various inorganic substances, usually individual elements in the form of ions, which constitute about 4% of the total body weight; they are concentrated most densely in the skeleton; they can be divided into two categories: (1) those present in relatively large amounts, e.g., sodium, potassium, calcium, magnesium, chlorine, phosphorous, and sulphur, and (2) trace elements - those present at a concentration of less than 50mg/kg, e.g., selenium; some are also considered "essential," i.e., those essential to growth or health, e.g. sodium and selenium; they may interact with each other on a variety of levels: (1) as co-factors in enzymes, they modulate the substrate/product relationship of biochemical pathways, (2) as ions across membranes, they contribute to: (a) the state of electrical excitability, e.g., the action potential, (b) the distribution of fluid volumes between fluid compartments, (c) competitive interactions, e.g., of zinc and copper for intestinal absorption, and (d) as common targets for regulatory compounds, e.g., vitamin D's action on calcium, phosphate and magnesium.
vitamins
Any of various fat-soluble or water-soluble organic substances essential in minute amounts for normal growth and activity of the body and obtained naturally from plant and animal foods; most vitamins, or their immediate metabolic derivatives, serve as co-enzymes or co-factors in a variety of enzyme catalyzed metabolic reactions; since they are recycled many times in these reactions before becoming useless, only small quantities are required by the body to replace those lost in each period of time.
oil soluble vitamins
(A, D, E, K) aka - fat-soluble vitamins
water soluble vitamins
(B complex, C)
vitamin A
One of the four oil-soluble [aliphatic alcohol] vitamins, occurring in nature in two forms: retinol and dehydroretinol; it is found in fish liver oils, liver, butter, egg yolk, cheese and most yellow and orange fruits and vegetables, in most of which it exists in its precursor form, carotene; in the developing world, most vitamin A comes from vegetable sources whereas in the developed world, animal sources are the main dietary source; Vitamin A is formed from carotene (and other provitamins) in the small intestine; bile salts and fat are required for its absorption; after absorption, vitamin A is transported in chylomicrons to the liver, where it is either stored as an ester, or re-exported to critical tissue sites in combination with retinol binding protein; vitamin A is necessary for the normal growth of epithelial tissue, aids in the synthesis of polysaccharides, is essential for formation of the visual pigment, rhodopsin, and it may have a role in protection against degenerative and neoplastic tissue changes.
B complex vitamins
A range of water-soluble vitamins with little structural similarity; they are grouped together because (1) they all act as coenzymes which participate in various metabolic reactions, and (2) they all tend to assimilate together in similar foods, e.g., milk and cereals; the group includes: vitamin B1 - thiamine, vitamin B2 - riboflavin, vitamin B6 - pyridoxine, vitamin B12 - cyanocobalamin, niacin, pantothenic acid, folate, and biotin.
vitamin C
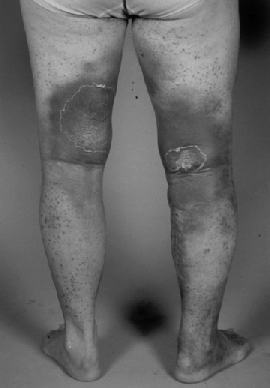
A small, water soluble organic compound, ascorbic acid, which promotes many metabolic reactions, particularly the laying down of collagen during the formation of connective tissue; it does this by facilitating the activity of enzymes which hydroxylate lysine, proline and procollagen; hence, it promotes wound healing; it also has vital roles in maintaining folate as tetrahydrofolate and in the absorption of iron; sources of vitamin C include citrus fruits, tomatoes, green vegetables, kidney, liver and milk; cooking of these source reduces the vitamin C content by virtue of oxidation and absorption into the cooking water; absorption takes place within the ileum by secondary active transport along with sodium ions; vitamin C deficiency produces classical scurvy, a condition characterized by general weakness, anemia, gum disease (gingivitis), and skin hemorrhages, scurvy is now most frequently seen in older, malnourished adults and is very rare in developed countries; Vitamin C status is determined from an assay on white blood cells. aka - ascorbic acid.
Image:Scurvy
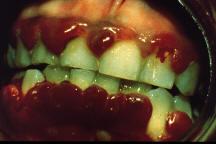
Image: Scurvy
vitamin D
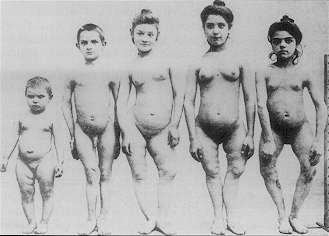
One of the four oil-soluble vitamins; a collective term for a range of oil- or fat-soluble compounds (e.g., vitamin D2 = ergocalciferol, pro-vitamin D3 = 7-dehydrocholesterol, vitamin D3 = cholecalciferol, 25-hydroxycholecalciferol = 25-HCC = calcidiol, 1,25-dihydroxycholecalciferol = 1,25-DHCC = calcitriol) with similar activity on calcium and phosphate physiology: (1) the enhancement of gastrointestinal absorption and (2) an increase in mineral deposition in bone, in nature, cholecalciferol is supplied principally through the action of ultraviolet light on skin; there are very few natural foods which contain substantial amounts of vitamin D in either the D2 or D3 form - they include fish-liver oils, egg yolk and fortified milk, margarines and breakfast cereals; ergosterol within plant matter can be converted to vitamin D; the consequences of vitamin D deficiency are (1) lowered blood levels of calcium and phosphate producing a secondary hyperparathyroidism in response, and (2) a further reduction in phosphate levels and increased plasma levels of bone-derived alkaline phosphatase; clinically, these produce poor bone mineralisation - rickets in children, osteomalacia in adults; depending on the cause, there may be a rapid response to administration of small doses of calciferol.
Image: Rickets
vitamin E
One of the four oil-soluble vitamins, an oil- or fat-soluble dietary vitamin necessary for normal reproduction, muscular development, resistance of erythrocytes to hemolysis, and various other biochemical functions in humans and animals; also, it can act as an antioxidant; a collective term for a range of eight naturally occurring oil- or fat-soluble compounds (the tocopherols and tocotrienols, of which, d-alpha-tocopherol is the most widely available and the most biologically active); sources of vitamin E include fresh nuts, wheat germ seed oils, and green leafy vegetables; animal products are generally poor sources; absorption depends on the digestion and absorption of fat; free tocopherols passively enter the lymphatic circulation, whereas metabolites and a small amount of vitamin E enter the portal circulation; when circulating in the blood, it is principally bound to low-density and high-density lipoprotein (LDL and HDL); it is stored in the liver, adipose tissue, muscle, pituitary gland, testes and adrenals.
vitamin K
One of the four oil-soluble vitamins; a collective term for a range of oil- or fat-soluble organic compounds: (1) vitamin K1 = the phylloquinones, present in green vegetables and fruit, and (2) vitamin K2 = the menaquinones, synthesized by bacteria within the gut; vitamin K plays an essential role in blood clotting by acting as a cofactor for the post-translational carboxylation of glutamate residues in clotting factors II (prothrombin), VII, IX and X by the liver; bile salts and fat are required for its absorption, and triglyceride-rich lipoproteins (VLDLs) seem to be the major carriers of vitamin K in the plasma; it is stored in the liver and spleen; dietary sources include leafy vegetables such as spinach, cabbage and cauliflower, certain legumes, and some vegetable oils such as rapeseed and soyabean oils.
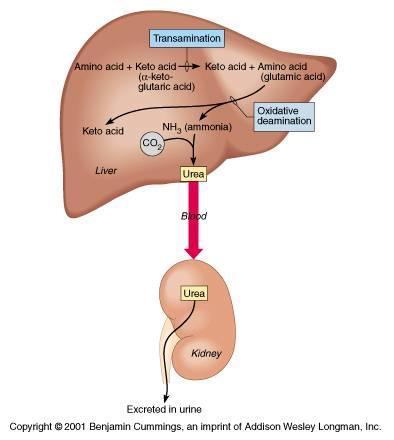
Describe: the relationship between deamination of amino acids and the formation of urea in the liver.
The liver has the major responsibility for maintaining the appropriate levels of the approximately twenty amino acids required by the body for protein synthesis and other functions. When a particular amino acid is present in the liver in excess of body needs, it may be transformed into some other needed amino acid by the process of transamination or it may be catabolized for energy by the process of deamination. It is necessary to remove all the -NH2 amino groups from the amino acid so that the remaining carbon "skeletons" can by transferred to one or another of the central carbohydrate catabolic pathways, glycolysis or the Kreb's Cycle = TCA Cycle = Citric Acid Cycle. The amino groups cannot be further broken down for any useful chemical energy, and if left as is, would spontaneously combine with water to for a dangerous strong base, ammonium hydroxide NH4OH. To prevent that outcome, enzymes in the liver immediately combine any amino groups generated from protein or nucleic acid catabolism or any ammonium ions absorbed from the large intestine which were by-products of normal flora bacterial metabolism with carbon dioxide molecules to produce a safe, highly water-soluble non-toxic nitrogenous waste compound, urea, which can be transported safely in the blood plasma to the kidneys for excretion. This reaction is 2CO2 + NH3 Û urea.
Diagram: the relationship between deamination of amino acids and the formation of urea in the liver.
Here are some reasonable cautions about vitamin and mineral supplementation:
Vitamin A: Excess amounts accumulate and can be toxic. Too much A can blur vision, cause headaches and vomiting, and also lead to liver, bone and central nervous system problems, among others.
Beta Carotene: The body converts this into vitamin A. One study found that high levels of beta carotene in the blood were linked to three times the risk of aggressive prostate cancer.
Vitamin C: There's no conclusive evidence that it prevents colds, heart disease, cataracts or cancer.
Vitamin E: Large doses can thin the blood and may increase the risk of hemorrhagic stroke in those with uncontrolled blood pressure. Has not been proven to protect the heart or prevent cancer.
Selenium: Most Americans get enough of this trace mineral in their diet. One study suggests that adding more via a pill may increase the risk of developing type 2 diabetes.
Folic Acid: It's a must during pregnancy to help prevent birth defects, but recent studies show no real effect for the rest of us against heart disease, cancer or depression. The connection between folate and reduced risk of Alzheimer's is not yet conclusive either.
Niacin: This B vitamin can be used to treat high cholesterol, but only under a doctor's supervision due to the risk of potentially serious side effects, including liver damage.
Lycopene: Two studies, one by the FDA, recently concluded that consuming lycopene as a supplement or in rich food sources, such as tomatoes, does not offer strong cancer-fighting protection, as was
Diagram: Krebs Cycle

Krebs cycle = citric acid cycle
A repetitive series of enzymatic reactions occurring in the matrix of the mitochondria involving oxidative metabolism of acetyl grouped, provided by the production of Acetyl CoA from the decarboxylattion of pyruvate molecules to produce high-energy phosphate compounds (GTPs) or electron transport compounds (NADs and FADs) capable of transferring energetic electrons and hydrogen ions to the electron transport system to produce high-energy phosphate compounds (GTPs); the products of this pathway are a major source of cellular energy. aka - tricarboxylic acid cycle.
pyruvate = pyruvic acid
The 3-carbon end product of aerobic glycolysis which can be transferred from the cell cytoplasm to the matrix of the mitochondria to enter the citric acid cycle, which can use the molecule as a nutrient source for additional energy production.
lactate = lactic acid
The 3-carbon end product of anaerobic glycolysis (fermentation of pyruvate) which must leave the muscle cell and travel to the liver, heart, or kidneys, which can use the molecule as a nutrient source for additional energy production; the liver is also capable of reconverting this molecule into glucose or glycogen.
acetyl CoA
A metobolic intermediary compound consisting of the 2-carbon acetate group covalently joined to the carrier CoEnzyme A; it carries acetate groups from the decarboxylation of pyruvate molecules or from the beta oxidation of fatty acids or from the deamination of amino acids to enter the citric acid cycle in the matrix of the mitochondria to produce useful chemical energy; this compound can also serve as a building block for the synthesis of fatty acids, amino acids, glucose, and other organic molecules in the cell.
List: the four stages of glucose catabolism and where they occur within the cell.
(1) glycolysis = the glycolytic pathway - occurs in the cytoplasm
(2) decarboxylation of pyruvate to acetate = formation of acetyl Coenzyme A - occurs in the matrix of the mitochondrion
(3) Kreb's Cycle = Citric Acid Cycle = TriCarboxylic Acid Cycle - occurs in the matrix of the mitochondrion
(4) oxidative phosphorylation = electron transport chain - occurs in the inner membrane = cristae membrane of the mitochondrion
SKetch and Label:the structure of a mitochondrion. Show where the four stages of glucose catabolism occur relative to the structure of the mitochondrion.
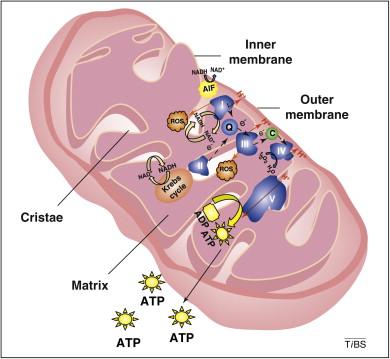
1) glycolysis = the glycolytic pathway - occurs in the cytoplasm
(2) decarboxylation of pyruvate to acetate = formation of acetyl Coenzyme A - occurs in the matrix of the mitochondrion
(3) Kreb's Cycle = Citric Acid Cycle = TriCarboxylic Acid Cycle - occurs in the matrix of the mitochondrion
(4) oxidative phosphorylation = electron transport chain - occurs in the inner membrane = cristae membrane of the mitochondrion
Diagram: the parts of cellular respiration

Describe: the fate of pyruvate under (a) anaerobic and (b) aerobic conditions in the cell in terms of the final end (waste) products and useful energy harvest (ATP production).
1. Anaerobic;
Final End (Waste) Products:2 molecules lactate per glucose and waste heat
Useful Energy Harvest: (ATP Production: net gain 2 ATPs per glucose
2. Aerobic;
Final End (Waste) Products: 6 CO2 and 12 H2O and waste heat
Useful Energy Harvest: net gain 36-38 ATPs per glucose
glycerol
An intermediary metabolite and a structural component of many biologically important lipids including neutral fats (mono-, di-, and tri-glycerides) and phospholipids; it consists of a 3 carbon frame to which three hydroxyl groups (-OH) are attached, making it a triatomic alcohol.
ketone bodies
Any of a class of organic compounds, such as acetone, having a carbonyl group linked to a carbon atom in each of two hydrocarbon radicals and having the general formula R(CO)R, where R may be the same as R ; in general they are colorless volatile liquids having a pungent odor; in lipid metabolism, three of the byproducts of fatty acid catabolism (acetone, acetoacetate, and beta-hydroxybutyrate). aka - ketones
ketogenesis
The metabolic production of ketone bodies (acetone, acetoacetate, and beta-hydroxybutyrate) which are byproducts of fatty acid catabolism; they may accumulate in excess in the blood and urine of uncontrolled or out-of-control diabetics because the majority of cells in their bodies are unable to utilize glucose for fuel despite its presence.
ketosis = ketoacidosis
An acidosis (an abnormal increase in the acidity, i.e., hydrogen ion concentration, in the body fluids) with an accumulation of ketone bodies (acetone, acetoacetate, and beta-hydroxybutyrate); it occurs primarily in untreated or out-of-control diabetes mellitus or in starvation as a byproduct of increased fatty acid catabolism.
acidosis
Any abnormal increase in the acidity of the body's fluids (with a corresponding drop in the pH), caused either by the accumulation of acids (increase in hydrogen ions) or by depletion of bicarbonate ions which serve as buffers; there are a variety of specific causes which fall into two main groups, respiratory and metabolic.
cholesterol
A multiple (4-membered) carbon-ring lipid molecule which serves as a minor structural component in cell membranes and is also the precursor for synthesis of estrogen, testosterone, and related steroid hormones
lipoproteins
Any of the series of soluble lipid-protein complexes which are transported in the blood; each aggregate particle consists of a spherical hydrophobic core containing triglycerides and cholesterol esters surrounded by an amphipathic monolayer of phopholipids, cholesterol and apolipoproteins; classes of lipoproteins include chylomicrons, very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL).
chylomicrons
The class of largest diameter soluble lipid-protein complexes which the lowest in density (mass to volume ratio); their composition is ~2% apolipoproteins, ~5% cholesterol, and ~93% triglycerides and phospholipids; their normal role is to be synthesized by the intestinal mucosal cells to transport dietary (exogenous) triglycerides and other lipids from the intestines via the lacteals and lymphatic system to the systemic circulation to the adipose tissue and liver for storage and use; they are only present in the blood in significant quantities after the digestion of a meal.
high-density lipoproteins (HDL)
class of small diameter soluble lipid-protein complexes which the highest in density (mass to volume ratio); their composition is ~45% apolipoproteins, ~25% cholesterol, and ~30% triglycerides and phospholipids; their normal role is to transport cholesterol and other lipids from the tissues to the liver for disposal; elevated levels of HDL are associated with decreased risk of cardiovascular disease.
low-density lipoproteins (LDL)
The class of large diameter soluble lipid-protein complexes which the fourth lowest in density (mass to volume ratio); their composition is ~25% apolipoproteins, ~45% cholesterol, and ~30% triglycerides and phospholipids; their normal role is to transport cholesterol and other lipids from the liver and intestines to the tissues for use; elevated levels of LDL are associated with increased risk of cardiovascular disease. nickname - bad cholesterol
List: 4 types of lipoproteins and their functions.
1.chylomicrons: transport lipids absorbed from a meal from the intestines to the adipose tissue and, to a lesser degree, to the liver, for storage
2.very low-density lipoproteins (VLDL): transport lipids, particularly neutral fats (triglycerides) and cholesterol, from the the liver to most somatic tissue cells for their various metabolic purposes
3.intermediate-density lipoproteins (IDL): transport lipids, neutral fats (triglycerides) and cholesterol, from the the liver to most somatic tissue cells for their various metabolic purposes
4.low-density lipoproteins (LDL): transport lipids, particularly cholesterol, and neutral fats (triglycerides), from the the liver to most somatic tissue cells for their various metabolic purposes
5.high-density lipoproteins (HDL): transport lipids, particularly cholesterol, and neutral fats (triglycerides), to the the liver for catabolism and elimination
[Note:][all lipoproteins transport smaller quantities of phospholipids which may be delivered to various cells for their various metabolic purposes, as well as serving, along with apolipoproteins, as the external emulsifiers of the lipoprotein droplets]
Sketch and Label: a simplified diagram of the structure of a lipoprotein.

The main points to note for the exam are these: (1) Any lipoprotein consists of an inner hydrophobic core and an outer layer of amphipathic molecules, one molecule thick, which serves to emulsify the lipoprotein droplet in the water of the body fluids; (2) the outer layer consists primarily of phospholipids and free cholesterol molecules and a smaller number of characteristic proteins called apolipoproteins; (3) the inner core consists of large quantities of hydrophobic lipids, neutral fats = triglycerides and cholesterol esters; (4) the differences among the different classes of lipoproteins consist primarily of differences in the proportions of lipids in the core as well as some differences in the apolipoproteins present in the outer layer.
cellular respiration
The oxidative process occurring within living cells by which the chemical energy of organic molecules is released in a series of metabolic steps with the production of ATP, some occurring in the cytoplasm and some within the mitochondria, involving the consumption of oxygen and the liberation of carbon dioxide and water; it is generally divided into two stages: (1) anaerobic glycolysis in the cytoplasm followed by (2) the citric acid cycle and oxidative phosphorylation at the electron transport chain in the mitochondria. aka - aerobic respiration
anaerobic
(1) a process not depending on free oxygen or air, e.g., anaerobic glycolysis; (2) living or occurring only in the absence of oxygen, e.g., anaerobic bacteria.
aerobic
(1) a process depending on free oxygen or air, e.g., aerobic glycolysis; (2) involving or improving oxygen consumption by the body, e.g., aerobic exercise; (3) living or occurring only in the presence of oxygen, e.g., aerobic bacteria.
oxidation
Any chemical reaction in which the atoms in an element lose electrons and the valence of the element is correspondingly increased; the products of such reactions tend to have less potential chemical energy; often, but not always, the loss of electrons is caused by the addition of an oxygen atom to the molecule; they always occur coupled to reduction reactions.
reduction
Any chemical reaction in which one or more hydrogens is combined with a compound or in which one or more oxygens is removed from a compound; is such reactions, there is a decrease in the positive valence or an increase in negative valence by the gaining of electrons; the products of such reactions tend to have more potential chemical energy; they always occur coupled to oxidation reactions.
oxidation reduction reactions
Any pair of coupled chemical reactions, one an oxidation and the other a reduction, in which one or more electrons are transferred from one atom or molecule to another atom or molecule, changing the oxidation number (valence) of both; the electron donor becomes oxidized and its oxidation number (valence) increases; the electron acceptor becomes reduced and its oxidation number (valence) decreases; energy is transferred in the process from the oxidized compound, the electron donor, to the reduced compound, the electon acceptor. Nickname - redox reactions.
dehydrogenation reactions
A diverse group of biological oxidation reactions in which one or more hydrogen ions or protons are removed from a molecule along with the removal of useful chemical energy in the form of energetic electrons which will be transferred to some other molecule(s) in a coupled reduction reaction; these reactions are usually catalyzed by dehydrogenase enzymes; in living organisms, cellular respiration is actually a process of oxidation of nutrients wherein some steps involve dehydrogenation.
electron carriers
A diverse group of intermediary compounds, often coenzymes derived by modification from certain ingested water-soluble vitamins, and often joined to nucleotide containing a nitrogenous base, e.g., adenine, which participate reversibly in oxidation reduction reactions and take part by transferring energetic electrons removed from some oxidized substrate and delivering them to another molecule which will become a reduced product of the coupled reaction; e.g., Nicotinamide Adenine Dinucleotide (NAD), Nicotinamide Adenine Dinucleotide Phosphate (NADP), Flavin Mono-Nucleotide (FMN), and the Flavin Adenine Dinucleotide (FAD) and the quinones, e.g., Coenzyme Q, found within the lipid membrane in mitochondria.
coenzymes
Any of the non-protein organic substances which usually contains a vitamin or mineral and are frequently phosphorylated, which combine with a specific protein, the apoenzyme, to form an active enzyme system by activating the apoenzyme protein, e.g., Adenosine Triphosphate (ATP), Coenzyme A, and many of the electron carriers, e.g., Nicotinamide Adenine Dinucleotide (NAD), Nicotinamide Adenine Dinucleotide Phosphate (NADP), Flavin Mono-Nucleotide (FMN), and the Flavin Adenine Dinucleotide (FAD).
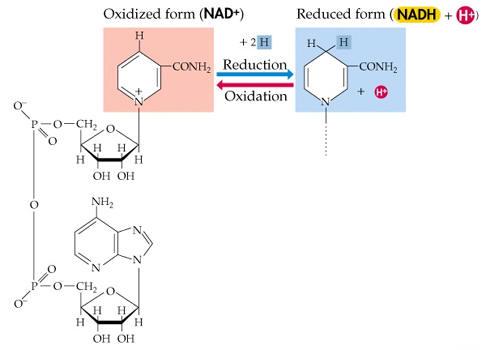
Nicotinamide Adenine Dinucleotide (NAD)
A coenzyme, C21H27N7O14P2, derived from the B vitamin nicotinic acid; an electon carrier and hydrogen carrier occurring in most living cells, and utilized alternately with NADH as an oxidizing or reducing agent in various metabolic processes, e.g., in various steps in the oxidation of glucose.
Diagram: NAD
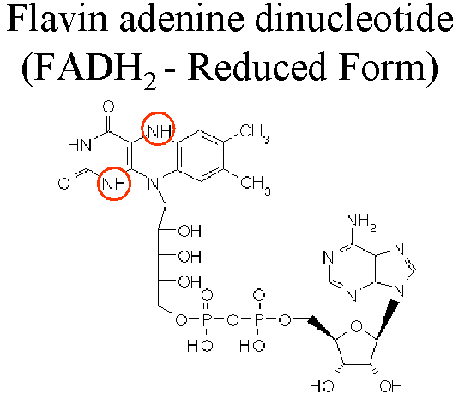
Flavin Adenine Dinucleotide (FAD
A coenzyme, C27H33N9O15P2, derived from the B vitamin riboflavin; an electon carrier and hydrogen carrier occurring in most living cells, and utilized alternately with FADH as an oxidizing or reducing agent in various metabolic processes, e.g., in certain step in the oxidation of glucose within the citric acid cycle.
Diagram: FAD (oxidized form)

Diagram FADH2 (reduced form)
electron transport chain
A series of membrane-bound electron carriers, various quinones, iron-sulfur (FeS) proteins, and cytochromes, located in the inner cristae membrane of mitochondria which receive energetic electrons from various electron carriers, and various related membrane-bound dehydrogenase enzymes, which use that energy in a stepwise series of oxidation reduction reactions to create a proton motive force (chemiosmosis) which pumps hydrogen ions from the matrix of the mitochondria to the intermembrane space which will ultimately be used by ATP Synthetase to generate ATPs. aka - electron transport system, cytochrome chain, cytochrome system.
List:the functions of coenzymes in intermediary metabolism.
Most are electron transport compounds, metabolic "shuttles" for singles or pairs of energetic electrons (usually with H+ ions traveling with them), which transfer these electrons from substrate molecules to product molecules in enzyme catalyzed oxidation-reduction reactions.
List: three specific examples of oxidation-reduction.
(1) C6H12O6 + 6O2 Û 6CO2 + 6H2O + 36 ATP + waste heat
(2) NAD+ + 2e- + 2H+ Û NADH+ + H+
(3) pyruvate + NADH+ + H+ Û lactate + NAD+
[Note: There are many other specific examples in metabolism, including many in fundamental glucose metabolism.]
Sketch and Label:a simplified diagram of the four stages of glucose catabolism. Account for the CO2, electrons (= hydrogens), and ATP extracted or produced in each stage, accounting for the energy yield from one glucose.
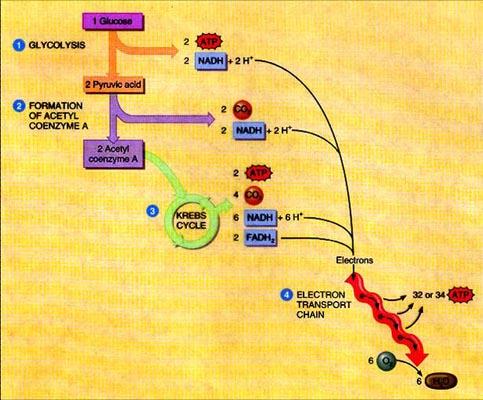
(1) Glycolysis: no CO2, net gain two ATPs, 2 electron pairs (NADH+ + H+)
(2) Formation of Acetyl Coenzyme A: two CO2, no ATP, 2 electron pairs (NADH+ + H+)
(3) Kreb's Cycle: four CO2, two ATPs, 6 electron pairs (NADH+ + H+) and 2 electron pairs (FADH+ + H+)
(4) Electron Transport Chain: no CO2, 32 or 34 ATPs, no electron pairs
phosphorylation
Any chemical reaction which adds a phosphate group (HPO4-) to an organic molecule; to create the covalent bond, chemical energy of some form must be provided; in human cells, such reactions form two categories: (1) substrate-level and (2) oxidative.
substrate-level phosphorylation
Synthesis of high energy phosphate compounds through the enzyme catalyzed (kinases) addition of low energy phosphate ions to an activated substrate, usually an organic molecule; these substrate-level phosphorylation reactions are coupled to energy yielding reactions, e.g., ATP hydrolysis, which occur in the cytoplasm of various cells; an example is the phosphorylation of creatine by ATP, catalyzed by creatine kinase, which occurs in striated muscle tissue.
oxidative phosphorylation
The phosphorylation of adenosine diphosphate (ADP) and synthesis of adenosine triphosphate (ATP) by the complex of oxidation reduction reactions localized in the electron transport chain and ATP synthetase enzymes of the inner christae membranes of the mitochondria which use the energy derived from the oxidation of nutrients such as glucose or fatty acids and delivered to the system by electron transport molecules such as FAD and NAD.
kinase
Any of various enzymes which catalyze the transfer of a phosphate group from a donor molecule, such as ADP or ATP, to an acceptor molecule; such transfers also often involve the transfer of useful chemical energy with which the cell can do work.
ATP synthase (= ATP synthetase)
An enzyme which catalyzes the covalent linking together of adenosine diphosphate (ADP) and a phosphate ion to form adenosine triphosphate (ATP) by using the energy derived from the oxidation of nutrients such as glucose or fatty acids; the enzyme is located within the inner membrane of the mitochondria and its synthetic activity is directly tied to the proton motive force (chemiosmosis) generated by the electron transport chain which receives energetic electrons from electron carriers NAD and FAD, and uses that energy to pump hydrogen ions from the mitochondrial matrix to the intermembrane space, and when those hydrogen ions follow their concentration gradient back to the matrix, they pass through a channel in this enzyme and provide the immediate source of energy for the synthesis of the ATP.
creatine phosphate
The small organic compound found in muscle cells which serves as a molecular storage depot for chemical energy in the form of high energy phosphate groups; when needed this compound transfers a high energy phosphate group to ADP to form ATP.
chemiosmosis
The theoretical mechanism which explains energy transduction (the proton motive force) in the mitochondrion; as a general mechanism, it is the coupling of one enzyme catalysed reaction to another using the transmembrane flow of an intermediate species; for example, cytochrome oxidase pumps protons across the mitochondrial inner cristae membrane and ATP synthesis is driven by re-entry of protons through the ATP synthesizing protein complex.
photophosphorylation
Phosphorylation, specifically ATP synthesis, induced by radiant sunlight energy during photosynthesis which is carried out by green plants and certain photosyntheitc microorganisms.
List:the three main types of phosphorylation
substrate-level phosphorylation, oxidative phosphorylation, photophosphorylation
1/2 Describe: the electron transport system and its relation to ATP synthetase.
The Electron Transport System (ETS) consists of a series of complexes which are clusters of related oxidation-reduction enzymes and associated membrane-bound electron-transfer compounds which are located as integral proteins within the inner = cristae membrane of the mitochondrion. See the figure below.
Complex I is capable of accepting the most energetic pairs of electrons which are carried by NAD+. As these electron pairs are transferred along the series of oxidation-reduction enzymes in the ETS pathway, some useful chemical energy is extracted from them.
Because FAD+ carries somewhat less energetic pairs of electrons, those electrons must be delivered to the ETS pathway beyond Complex I and, therefore, somewhat less useful chemical energy can be extracted from them as the complete the path.
All of the useful chemical energy extracted from these pairs of energetic electrons is used to power the active transport of hydrogen ions from the matrix in the interior of the mitochondrion to the intermembrane space. See the figure to the right.
This extensive hydrogen pumping establishes a powerful electrochemical gradient favoring the movement of the hydrogen ions back across the inner = cristae membrane of the mitochondrion. This gradient, its potential energy, is the proton motive force.
In the mitochondrion, the only route for hydrogen ions to return to the mitochondrial interior, the matrix, is by passing through the enzyme ATP synthetase. In so doing, the pro
2/2 Describe: the electron transport system and its relation to ATP synthetase.
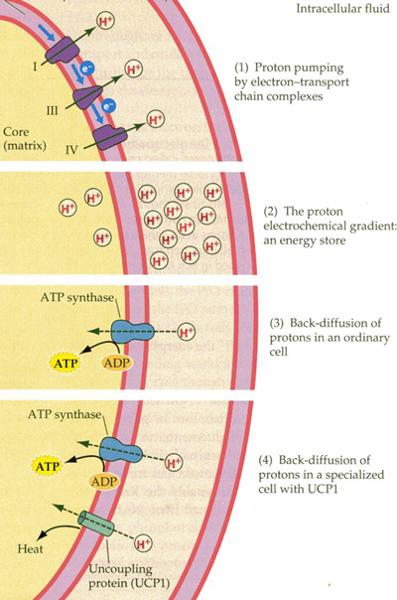
Describe:chemiosmosis
Chemiosmosis is the theoretical mechanism which explains energy transduction (the proton motive force) in the mitochondrion; as a general mechanism, it is the coupling of one enzyme catalysed reaction to another using the transmembrane flow of an intermediate species; for example, cytochrome oxidase pumps protons across the mitochondrial inner cristae membrane and ATP synthesis is driven by re-entry of protons through the ATP synthesizing protein complex. See also the discussion in question 8 above.
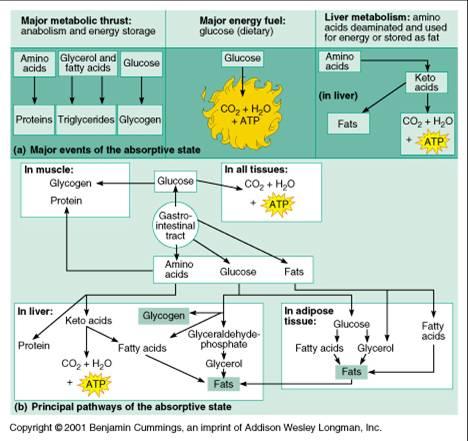
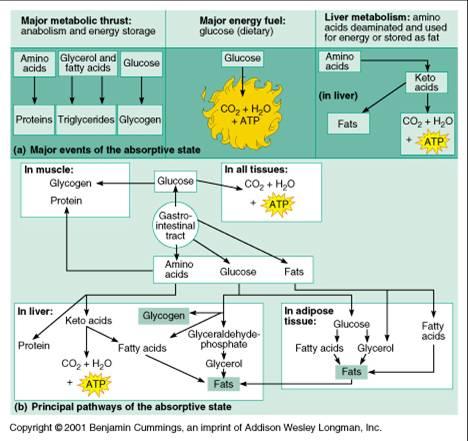
1/2 absorptive state
That period of time in the body during which cell metabolism is primarily fueled by nutrients derived by absorption from the most recent meal; this state is dominated by insulin release from the beta cells of the pancreatic islets in response to hyperglycemia; most cells in the body are responsive targets to insulin and, therefore, in response to insulin arrival, extract glucose from the plasma to use in oxidative pathways to generate ATP; the liver and skeletal muscle responds to insulin in an additional way, storing glucose as glycogen (glycogenosis); most other nutrients from the meal are routed to the liver via the hepatic portal system where they are processed and stored, however, fats are routed to the general circulation via the lacteals and lymphatic drainage, where they are processed and stored as neutral fats (lipogenesis); nervous tissue is not responsive to insulin, but does utilize glucose for fuel use in oxidative pathways to generate ATP; non-nervous tissue will also respond to insulin by taking up and using plasma amino acids for protein synthesis as needed; this time period usually lasts from approximately two hours after the meal was ingested to four hours after the meal was ingested; during this same period, digestive activities are being regulated by the hormones gastrin, secretin, and cholecystokinin, etc.
2/2 absorptive state
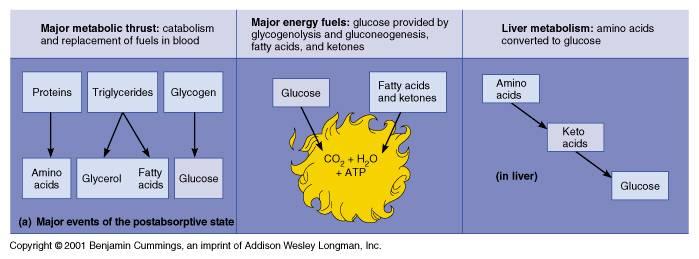
1/3 postabsorptive state
That period of time in the body during which cell metabolism is primarily fueled by nutrients stored from previous meals; this state is dominated by glucagon release from the alpha cells of the pancreatic islets in response to hypoglycemia; various "insulin antagonists," thyroid hormones (T3 and T4), glucocorticoids (cortisone, cortisol, hydrocortisone) from the adrenal cortex, epinephrine from the adrenal medulla, and human growth hormone from the anterior pituitary = adenohypophysis are also present in increased amounts and contribute to the physiological processes which occur in this state; primarily, hepatocytes are the responsive targets to glucagon and, in response to glucagon arrival, release glucose into the plasma to be used by nervous tissue in oxidative pathways to generate ATP; the liver obtains this glucose in two ways: (1) by releasing glucose which had been stored as glycogen (glycogenolysis) and (2) by synthesizing new glucose molecules from the breakdown products of lipid and protein catabolism (gluconeogenesis); if skeletal muscle tissue becomes active, it will also utilize glucose stored as glycogen (glycogenolysis); most other tissues shift to energy production from lipid and protein catabolism; adipocytes liberate fats into the bloodstream (lipolysis) to support the metabolsim of these other tissues, adipocytes may also use the glycerol from fat catabolism to synthesize glucose (gluconeogenesis); the liver will oxidize free fatty acids for fuel, producing
2/3 postabsorptive state
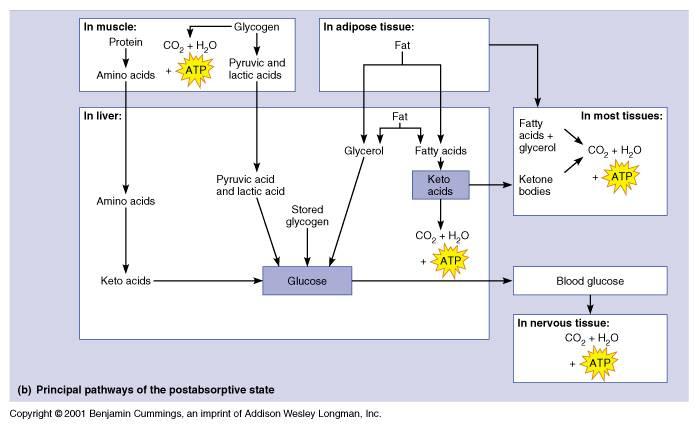
3/3 postabsorptive state
1/2 Describe: the regulation of nutrient usage for storage and/or energy production in the absorptive state.
Insulin dominates during the absorptive state. All tissues (including nervous tissue) respond by absorbing glucose from the blood and using it to fuel oxidative metabolism to generate ATPs. In addition, many tissues also absorb nutrients provided by the recent meal and store them as proteins and glycogen (liver, skeletal muscle, etc.,) or as neutral fats = triglycerides (liver, adipose tissue, etc.). The liver will also deaminate excess amino acids and convert them to keto acids which may be used for oxidative metabolism or stored as neutral fats = triglycerides
2/2 Describe: the regulation of nutrient usage for storage and/or energy production in the absorptive state.
Describe: the regulation of nutrient usage for storage and/or energy production in the postabsorptive state.
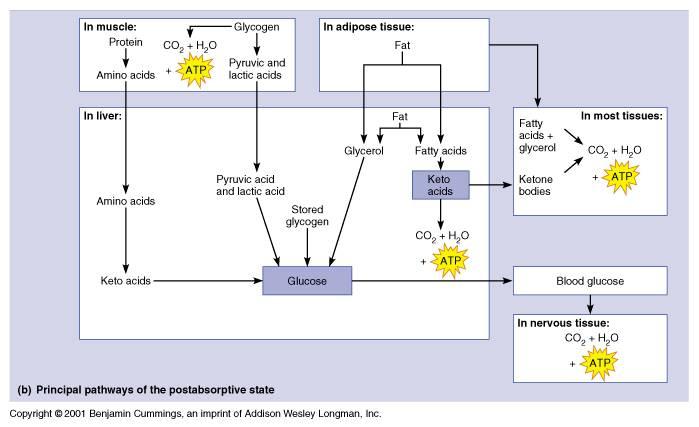
Glucagon, assisted by the group of insulin-antagonists (human growth hormone, thyroid hormones T3 & T4, glucocorticoids (cortisol, cortisone, hydrocortisone, etc.), and epinephrine = adrenalin, dominates during the post-absorptive state. Nervous tissue, which is unresponsive to insulin, continues to absorb glucose from the blood and using it to fuel oxidative metabolism to generate ATPs. To provide this glucose, the liver catabolizes proteins and lipids to synthesize new glucose molecules, the process of gluconeogenesis, as well as hydrolyzing glycogen, glycogenolysis, to liberate stored glucose. The glucose molecules from these two sources are passed into the blood stream to support the requirements of nervous tissue. All the other, insulin-responsive tissues of the body shift to the catabolism of neutral fats = triglycerides to fuel oxidative metabolism to generate ATPs. Both fat and protein catabolism generate ketone bodies as by-products which may also serve as fuels for oxidative metabolism to generate ATPs. The liver and adipose tissue provide the bulk of the fats used for oxidative metabolism in the post-absorptive state.
Describe: describe the time sequence between the absorptive and postabsorptive states during a typical 24 hour day.
The time sequences for absorptive and postabsorptive states during a typical 24 hour day are quite variable and depend on the size, content, and number of meals and snacks ingested, as well as the times of ingestion. As a general rule, the body enters the absorptive state approximately two hours after a normal full meal (as opposed to a snack), allowing time for initial digestion in the stomach, and remains in the postabsorptive state for approximately four hours while the nutrients from the meal are being absorbed from the chyme passed to the small intestine. After absorption is nearly complete and after nutrients, especially glucose, from the meal have been transferred to the cells of the various body tissues, then the body enters the post-absorptive state and continues in the post-absorptive state until the next meal is digested by the stomach and passed to the small intestine. thus, the body is typically in the post-absorptive state for the longest continuous period of time during a night's sleep. Individuals who snack frequently may never make a complete shift to the post-absorptive state during waking hours. This is not a problem for their physiology; in fact, it is simply appropriate to their digestive status.
insulin
The protein hormone secreted by the beta cells of the pancreatic islets of Langerhans; it reduces the concentration of glucose in the blood by targeting the liver to promote glycogenesis, i.e., the formation of glycogen from glucose, and it targets most cells of the body, other than nervous tissue, to take glucose from the blood and use it as metabolic fuel; its deficiency or failure to perform causes the disease diabetes mellitus.
glucagon
The protein hormone secreted by the alpha cells of the pancreatic islets of Langerhans; it is an insulin antagonist, raising the concentration of glucose in the blood by targeting the liver to promote glycogenolysis, i.e., the degradation of glycogen to glucose, and gluconeogenesis, the synthesis of new glucose from non-carbohydrate precursors; it uses cyclic AMP as its second messenger.
insulin antagonists
The group of regulatory substances which oppose the action of insulin (insulin lowers blood glucose levels and is most active during the absorptive state a few hours after a meal has been eaten); these regulatory substances elevate blood glucose levels by encouraging glycogenolysis, gluconeogenesis, and glucose release from the liver and they are most active during the post-absorptive state, from approximately four-five hours after the last meal until one-two hours after the next meal; these substances include the hormones glucagon (pancreas), human growth hormone (anterior pituitary), glucocorticoids (adrenal cortex), epinephrine (adrenal medulla), thyroid hormones (T3 & T4), etc.
glucocorticoids
class of steroid hormones produced by the middle layer of the adrenal cortex, whose release is stimulated by adenohypophyseal ACSH; examples include cortisol, cortisone, and hydrocortisone; the glucocorticoids are involved in carbohydrate, protein, and fat metabolism, and have anti-inflammatory properties; they are insulin antagonists contributing to increasing blood glucose levels and fat catabolism; they play a role in maintaining arterial blood pressue, alter the response of connective tissue to injury, reduce the number of circulating lymphocytes, and play a role in the functioning of the CNS.
cortisol
The main steroid hormone produced by the middle layer of the adrenal cortex, whose release is stimulated by adenohypophyseal ACSH; this glucocorticoid is involved in carbohydrate, protein, and fat metabolism, and has anti-inflammatory properties; it is aninsulin antagonist contributing to increasing blood glucose levels and fat catabolism; it plays a role in maintaining arterial blood pressue, alters the response of connective tissue to injury, reduces the number of circulating lymphocytes, and plays a role in the functioning of the CNS.
epinephrine = adrenalin
A catecholamine neurohormone derived from the amino acid tyrosine in the same pathway which produces norepinephrine, from which epinephrine is derived; it is released by the adrenal medulla and interacts with all target cells which have adrenergic receptors to ready the body for increased skeletal muscular activity or fight-or-flight emergencies.
human growth hormone (hGH) = somatotropin
A protein hormone secreted by the anterior lobe of the pituitary gland/adenohypophysis which targets most body tissues, particularly the liver, skeletal muscle, bone and cartilage, and promotes growth of the body, especially by stimulating release of somatomedins from the liver abd other tissues, and which stimulates protein catabolism; it is an insulin antagonist contributing to increasing blood glucose levels and fat catabolism.
feeding center = hunger center
Collections of neurons in the hypothalamus which evaluate and respond to changes in the blood levels of various nutritional metabolites, e.g., blood glucose, by issuing commands to brain stem centers that initiate behaviors leading to ingestion of foods
satiety center
The ventromedial region of the hypothalamus which is activated by hyperglycemia and by stretching sensations from the stomach to act to inhibit the feeding center of the hypothalamus, thus inhibiting food intake.
List:the factors which stimulate the hunger (feeding) center of the hypothalamus.
hypothalamic peptides (orexins, neuropeptide Y, galanin)
hypoglycemia and low plasma amino acid levels epinephrine
exposure to cold
psychological factors
List: the factors which stimulate the satiety center of the hypothalamus.
GLP-1 (glucagon-like peptide), serotonin
hyperglycemia and elevated plasma amino acid levels
elevated plasma fatty acid levels and leptin
insulin and CCK (cholecystokinin)
increased body temperature
psychological factors
List: 5 hormones which can be termed "insulin antagonists."
glucagon (pancreas)
human growth hormone (anterior pituitary)
glucocorticoids (adrenal cortex)
epinephrine (adrenal medulla)
thyroid hormones (T3 & T4)