If an African violet has chlorosis, which of the following elements might be a useful addition to the soil?
A. Copper
B. Iodine
C. Magnesium
D. Molybdenum
C. Magnesium
Large numbers of ribosomes are present in cells that specialize in producing which of the following molecules?
A. Proteins
B.Lipids
C. Glycogen
D. Nucleic acids
A. Proteins
In the process of carbon fixation, RuBP attaches a CO2 to produce a six-carbon molecule, which is then split to produce two molecules of 3-phosphoglycerate. After phosphorylation and reduction produces glyceraldehyde 3-phosphate (G3P), what more needs to happen to complete the Calvin cycle?
A. Regeneration of NADP+
B. Regeneration of ATP from ADP
C. Addition of a pair of electrons from NADPH
D. Regeneration of RuBP
D. Regeneration of RuBP
Which of the following does NOT occur during mitosis?
A. Condensation of the chromosomes
B. Separation of the spindle poles
C. Spindle formation
D. Replication of the DNA
D. Replication of the DNA
Asbestos is a material that was once used extensively in construction. One risk from working in a building that contains asbestos is the development of asbestosis caused by the inhalation of asbestos fibers. Cells will phagocytize asbestos, but are not able to degrade it. As a result, asbestos fibers accumulate in _____.
A. Peroxisomes
B. Lysosomes
C. Ribosomes
D. Mitochondria
B. Lysosomes
How do phospholipids interact with water molecules?
A. The polar heads avoid water; the nonpolar tails attract water (because water is polar and opposites attract).
B. The polar heads interact with water; the nonpolar tails do not.
C. Phospholipids do not interact with water because water is polar and lipids are nonpolar.
D. Phospholipids dissolve in water.
B. The polar heads interact with water; the nonpolar tails do not.
A chemical reaction that has a positive ΔG is best described as _____.
A. Exergonic
B. Spontaneous
C. Enthalpic
D. Endergonic
D. Endergonic
Which of the following statements is true of signal molecules?
A. In most cases, signal molecules interact with the cell at the plasma membrane, enter the cell, and eventually enter the nucleus.
B. Protein kinase A activation is one possible result of signal molecules binding to G protein-coupled receptors.
C. When signal molecules first bind to receptor tyrosine kinases, the receptors phosphorylate a number of nearby molecules.
D. In response to some G protein-mediated signals, a special type of lipid molecule associated with the plasma membrane is cleaved to form IP3 and calcium.
B. Protein kinase A activation is one possible result of signal molecules binding to G protein-coupled receptors.
Motor proteins provide for molecular motion in cells by interacting with what types of cellular structures?
A. Free ribosomes and ribosomes attached to the ER
B. Components of the cytoskeleton
C. Cellulose fibers in the cell wall
D. Membrane proteins of the inner nuclear envelope
B. Components of the cytoskeleton
White blood cells engulf bacteria using _____.
A. Pinocytosis
B. Osmosis
C. Phagocytosis
D. Receptor-mediated exocytosis
C. Phagocytosis
You are working on a team that is designing a new drug. For this drug to work, it must enter the cytoplasm of specific target cells. Which of the following would be a factor that determines whether the molecule selectively enters the target cells?
A. Similarity of the drug molecule to other molecules transported by the target cells
B. Hydrophobicity of the drug molecule
C. Lack of charge on the drug molecule
D. Lipid composition of the target cells' plasma membrane
A. Similarity of the drug molecule to other molecules transported by the target cells
You have a planar bilayer with equal amounts of saturated and unsaturated phospholipids. After testing the permeability of this membrane to glucose, you increase the proportion of unsaturated phospholipids in the bilayer. What will happen to the membrane's permeability to glucose?
A. Permeability to glucose will decrease.
B. Permeability to glucose will increase.
C. Permeability to glucose will stay the same.
D. You cannot predict the outcome. You simply have to make the measurement.
B. Permeability to glucose will increase.
Which of the following are water-conducting cells that are dead at functional maturity?
A. Tracheids and vessel elements
B. Sieve-tube elements
C. Collenchyma cells
D. Parenchyma cells
A. Tracheids and vessel elements
Which of the following is true for all exergonic reactions?
A. The products have more total energy than the reactants.
B. The reaction proceeds with a net release of free energy.
C. The reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants.
D. A net input of energy from the surroundings is required for the reactions to proceed.
B. The reaction proceeds with a net release of free energy.
Arrange the following five events in an order that explains the mass
flow of materials in the phloem.
1. Water diffuses into the sieve
tubes.
2. Leaf cells produce sugar by photosynthesis.
3.
Solutes are actively transported into sieve tubes.
4. Sugar is
transported from cell to cell in the leaf.
5. Sugar moves down
the stem.
2, 4, 3, 1, 5
4, 2, 1, 3, 5
2, 4, 1, 3, 5
1, 2, 3, 4, 5
2, 4, 3, 1, 5
The movement of water across biological membranes can best be predicted by _____.
A. Aquaporins
B. Prevailing weather conditions
C. Water potentials
D. Level of active transport
C. Water potentials
A gardener is concerned that her greenhouse is getting too hot from too much light and seeks to shade her plants with colored translucent plastic sheets, the color of which allows passage of only that wavelength. What color should she use to reduce overall light energy but still maximize plant growth?
A. Blue
B. Orange
C. Green
D. Any color will work equally well.
A. Blue
A controlled experiment _____.
A. Includes at least two groups, one of which does not receive the experimental treatment
B. Includes one group for which the scientist controls all variables
C. Includes at least two groups, one differing from the other by two or more variables
D. Is repeated many times to ensure that the results are accurate
A. Includes at least two groups, one of which does not receive the experimental treatment
Which of the following is true when comparing an uncatalyzed reaction to the same reaction with a catalyst?
A. The catalyzed reaction will be slower.
B. The catalyzed reaction will consume all of the catalyst.
C. The catalyzed reaction will have the same G.
D. The catalyzed reaction will have higher activation energy.
C. The catalyzed reaction will have the same G.
What is the primary function of stems?
A. Reproduction
B. Facilitation of gas exchange
C. Water absorption and movement
D. Maximization of photosynthesis by leaves
D. Maximization of photosynthesis by leaves
You have discovered an enzyme that can catalyze two different chemical reactions. Which of the following is most likely to be correct?
A. Either the enzyme has two distinct active sites or the reactants involved in the two reactions are very similar in size and shape.
B. The enzyme contains α-helices and β-pleated sheets.
C. Two types of allosteric regulation occur: The binding of one molecule activates the enzyme, while the binding of a different molecule inhibits it.
D. The enzyme is subject to competitive inhibition and allosteric regulation.
A. Either the enzyme has two distinct active sites or the reactants involved in the two reactions are very similar in size and shape.
Which of the following is the best description of a control for an experiment?
A. The control group is kept in an unchanging environment.
B. Only the experimental group is tested or measured.
C. The control group is matched with the experimental group except for one experimental variable.
D. The control group is exposed to only one variable rather than several.
C. The control group is matched with the experimental group except for one experimental variable.
What is the major structural difference between starch and glycogen?
A. The types of monosaccharide subunits in the molecules
B. The amount of branching that occurs in the molecule
C. The type of glycosidic linkages in the molecule
D. Whether glucose is in the α or β form
B. The amount of branching that occurs in the molecule
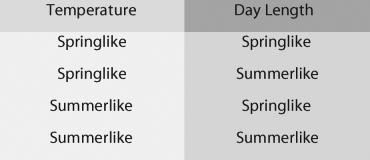
In each of the four environments, one of the caterpillars was fed oak
flowers, the other oak leaves. Thus, there were a total of eight
treatment groups (4 environments × 2 diets).
Refer to the
accompanying figure. Which one of the following is NOT a plausible
hypothesis to explain the differences in caterpillar appearance
observed in this population?
A. The cooler temperatures of spring trigger the development of flowerlike caterpillars.
B. The longer day lengths of summer trigger the development of twig-like caterpillars.
C. Differences in air pressure, due to differences in elevation, trigger the development of different types of caterpillars.
D. Differences in diet trigger the development of different types of caterpillars.
C.Differences in air pressure, due to differences in elevation, trigger the development of different types of caterpillars.
What is the difference between covalent bonds and ionic bonds?
A. Covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms.
B. Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
C. Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms.
D. Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C. Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms.
Which structure is NOT part of the endomembrane system?
A. Nuclear envelope
B. Plasma membrane
C. Golgi apparatus
D. Chloroplast
D. Chloroplast
Charles Darwin proposed a mechanism for descent with modification that stated that organisms of a particular species are adapted to their environment when they possess _____.
A. Heritable traits that decrease their survival and reproductive success in the local environment
B. Heritable traits that enhance their survival and reproductive success in the local environment
C. Non-heritable traits that enhance their survival in the local environment
D. Non-heritable traits that enhance their survival and reproductive success in the local environment
B. Heritable traits that enhance their survival and reproductive success in the local environment
The partial negative charge in a molecule of water occurs because _____.
A. One of the hydrogen atoms donates an electron to the oxygen atom
B. The electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus
C. The oxygen atom has two pairs of electrons in its valence shell that are not neutralized by hydrogen atoms
D. The oxygen atom donates an electron to each of the hydrogen atoms
B. The electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus
Which of the following would most likely be an immediate result of a growth factor binding to its receptor?
A. Protein phosphatase activity
B. Protein kinase activity
C. Phosphorylase activity
D. Adenylyl cyclase activity
B. Protein kinase activity
In what way are elements in the same column of the periodic table the same? They have the same number of _____.
A. Protons
B. Electrons in their valence shells when neutral
C. Electrons when neutral
D. Electron shells when neutral
B. Electrons in their valence shells when neutral
Which of the following statements about quorum sensing is FALSE? Quorum sensing _____.
A. Is particularly well studied because of its medical importance
B. Is species specific
C. Is cell-cell communication in eukaryotes
D. May result in biofilm formation
C. s cell-cell communication in eukaryotes
Which of the following cell types retains the ability to undergo cell division?
A. A stem fiber
B. A parenchyma cell near the root tip
C. A functional sieve tube element
D. A tracheid
B. A parenchyma cell near the root tip
Which criteria allow biologists to divide chemicals into macronutrients and micronutrients?
A. How they are used in metabolism
B. The quantities of each required by plants
C. Whether or not they are essential for plant growth
D. Molecular weight of the element or compound
B. The quantities of each required by plants
When oxygen is released as a result of photosynthesis, it is a direct by-product of _____.
A. The electron transfer system of photosystem I
B. The electron transfer system of photosystem II
C. Splitting water molecules
D. Chemiosmosis
C. Splitting water molecules
Most of the CO2 from the catabolism of glucose is released during _____.
A. Electron transport
B. The citric acid cycle
C. Glycolysis
D. Chemiosmosis
B. The citric acid cycle
Which two functional groups are always found in amino acids?
A. Carboxyl and amino groups
B. Carbonyl and amino groups
C. Hydroxyl and carboxyl groups
D. Amino and sulfhydryl groups
A. Carboxyl and amino groups
The tertiary structure of a protein is the _____.
A. Order in which amino acids are joined in a polypeptide chain
B. Overall protein structure resulting from the aggregation of two or more polypeptide subunits
C. Unique three-dimensional shape of the fully folded polypeptide
D. Organization of a polypeptide chain into an α-helix or β-pleated sheet
C. Unique three-dimensional shape of the fully folded polypeptide
Plasmodesmata in plant cells are most similar in function to which of the following structures in animal cells?
A. Tight junctions
B. Extracellular matrix
C. Gap junctions
D. Desmosomes
C. Gap junctions
Which of the following are compounds?
A. O2 and CH4
B. H2O, O2, and CH4
C. H2O and O2
D. H2O and CH4, but not O2
D. H2O and CH4, but not O2
Carbohydrates and fats are considered high-energy foods because they _____.
A. Are easily reduced.
B. Have no nitrogen in their makeup.
C. Have a lot of oxygen atoms.
D. Have a lot of electrons associated with hydrogen.
D. Have a lot of electrons associated with hydrogen.
Which chemical group can act as an acid?
A. Amino
B. Methyl
C. Carboxyl
D. Carbonyl
C. Carboxyl
When the atoms involved in a covalent bond have the same electronegativity, what type of bond results?
A. A hydrogen bond
B. A polar covalent bond
C. An ionic bond
D. A nonpolar covalent bond
D. A nonpolar covalent bond
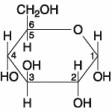
The molecule shown in the accompanying figure is _____.
A. A hexose
B. A pentose
C. Fructose
D. Maltose
A. A hexose
Which of the following descriptions best fits the class of molecules known as nucleotides?
A. A sugar and a purine or pyrimidine
B. A nitrogenous base and a sugar
C. A nitrogenous base and a phosphate group
D. A nitrogenous base, a phosphate group, and a sugar
D. A nitrogenous base, a phosphate group, and a sugar
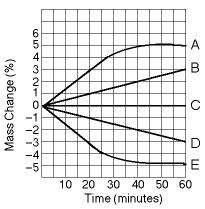
Five dialysis bags constructed of membrane, which is permeable to
water and impermeable to sucrose, were filled with various
concentrations of sucrose and then placed in separate beakers
containing an initial concentration of 0.6 M sucrose
solution. At 10-minute intervals, the bags were massed (weighed) and
the percent change in mass of each bag was graphed.
Which
line in the graph represents the bag with the highest initial
concentration of sucrose?
A. A
B. B
C. C
D. D
A. A
According to the induced fit hypothesis of enzyme catalysis, _____.
A. The binding of the substrate depends on the shape of the active site
B. The binding of the substrate changes the shape of the enzyme's active site
C. Some enzymes change their structure when activators bind to the enzyme
D. The active site creates a microenvironment ideal for the reaction
B. The binding of the substrate changes the shape of the enzyme's active site
The toxin of Vibrio cholerae causes profuse diarrhea because it _____.
A. Signals IP3 to act as a second messenger for the release of calcium
B. Binds with adenylyl cyclase and triggers the formation of cAMP
C. Modifies a G protein involved in regulating salt and water secretion
D. Modifies calmodulin and activates a cascade of protein kinases
C. Modifies a G protein involved in regulating salt and water secretion
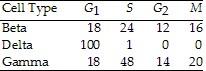
Use the data in the accompanying table to answer the following
question.
The data were obtained from a study of
the length of time spent in each phase of the cell cycle by cells of
three eukaryotic organisms designated beta, delta, and gamma.
Of the following, the best conclusion concerning the difference
between the S phases for beta and gamma is that _____.
A. Gamma contains more DNA than beta
B. Beta and gamma contain the same amount of DNA
C. Beta cells reproduce asexually
D. Beta is a plant cell and gamma is an animal cell
A. Gamma contains more DNA than beta
A solution contains 0.0000001 (10-7) moles of hydroxyl ions [OH-] per liter. Which of the following best describes this solution?
A. Basic: H+ acceptora
B. Acidic: H+ donor
C. Acidic: H+ acceptor
D. Neutral
D. Neutral
In comparison to eukaryotes, prokaryotes _____.
A. Are more structurally complex
B. Are smaller
C. Are larger
D. Do not have membranes
B. Are smaller
Substrate-level phosphorylation occurs _____.
A. In both glycolysis and the citric acid cycle
B. During oxidative phosphorylation
C. In the citric acid cycle
D. In glycolysis
A. In both glycolysis and the citric acid cycle
For living organisms, which of the following is an important consequence of the first law of thermodynamics?
A. The entropy of an organism decreases with time as the organism grows in complexity.
B. Organisms grow by converting energy into organic matter.
C. The organism ultimately must obtain all of the necessary energy for life from its environment.
D. The energy content of an organism is constant.
C. The organism ultimately must obtain all of the necessary energy for life from its environment.
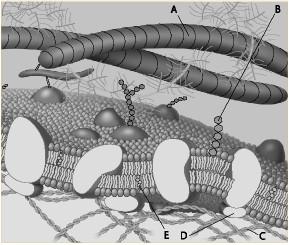
Which component is a microfilament (actin filament) of the cytoskeleton?
A. A
B. B
C. C
D. D
C. C
Why are hydrocarbons insoluble in water?
A. The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B. They exhibit considerable molecular complexity and diversity.
C. The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
D. They are less dense than water.
C. The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
Which of these is an example of inductive reasoning?
A. These organisms live in sunny regions. Therefore, they are using photosynthesis.
B. If two species are members of the same genus, they are more alike than each of them could be to a different genus.
C. Hundreds of individuals of a species have been observed and all are photosynthetic; therefore, the species is photosynthetic.
D. If protists are all single-celled, then they are incapable of aggregating.
C. Hundreds of individuals of a species have been observed and all are photosynthetic; therefore, the species is photosynthetic.
An animal cell lacking oligosaccharides on the external surface of its plasma membrane would likely be impaired in which function?
A. Establishing a diffusion barrier to charged molecules
B. Attaching the plasma membrane to the cytoskeleton
C. Transporting ions against an electrochemical gradient
D. Cell-cell recognition
D. Cell-cell recognition
You find yourself standing next to a beautiful rose bush. Which of the following do you and the rose have in common?
A. You both are multicellular.
B. You both lack a membrane-bound nucleus.
C. You and the rose have nothing in common.
D. You are both prokaryotic.
A. You both are multicellular.
Monocot vascular bundles do not have a vascular cambium between the xylem and phloem. This means that monocots _____.
A. Do not produce wood in annual rings
B. Have very thin stems
C. Are much less efficient at conducting water and sugars
D. Cannot produce lateral shoots
A. Do not produce wood in annual rings
Which of the following occurs in vascular land plants but not charophytes (stoneworts)?
A. Lignin
B. Cellulose
C. Chlorophyll a
D. Sporopollenin
A. Lignin
Gaucher disease is the most common of lipid storage diseases in
humans. It is caused by a deficiency of an enzyme necessary for lipid
metabolism. This leads to a collection of fatty material in organs of
the body including the spleen, liver, kidneys, lungs, brain, and bone
marrow.
Using your knowledge of the structure of
eukaryotic cells, identify the statement below that best explains how
internal membranes and the organelles of cells would be involved in
Gaucher disease.
A. The mitochondria are most likely defective and do not produce adequate amounts of ATP needed for cellular respiration.
B. The rough endoplasmic reticulum contains too many ribosomes which results in an overproduction of the enzyme involved in carbohydrate catalysis.
C. The Golgi apparatus produces vesicles with faulty membranes that leak their contents into the cytoplasm of the cell.
D. The lysosomes lack sufficient amounts of enzymes necessary for the metabolism of lipids.
D. The lysosomes lack sufficient amounts of enzymes necessary for the metabolism of lipids.
As an undergraduate research assistant, your duties involve measuring water potential in experimental soil-plant-atmosphere systems. Assume you make a series of measurements in a system under normal daylight conditions, with stomata open and photosynthesis occurring. Which of the following correctly depicts the trend your measurement data should follow if the cohesion-tension mechanism is operating?
A. Soil < roots = leaves < atmosphere
B. Soil < roots < leaves < atmosphere
C. Atmosphere < leaves = roots < soil
D. Atmosphere < leaves < roots < soil
D. Atmosphere < leaves < roots < soil
As an undergraduate research assistant, you are assisting with a radioisotope tracer experiment. You expose a mature leaf on one side of the lower shoot of a sugar beet plant to 14CO2 and then track the movement of the 14C atoms by radiography. Where are you LEAST likely to detect 14C?
A. The shoot apical meristem
B. A mature upper leaf on the opposite side of the plant from the treated leaf
C. The treated leaf
D. The roots
B. A mature upper leaf on the opposite side of the plant from the treated leaf
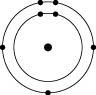
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
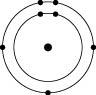
B
Which of these classes of biological molecules does NOT include polymers?
A. Carbohydrates
B. Lipids
C. Proteins
D. Nucleic acids
B. Lipids
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a molecule made by linking three glucose molecules together by dehydration reactions?
A. C18H32O16
B. C18H30O15
C. C18H36O18
D. C6H10O5
A. C18H32O16
Students conducted an experiment to determine the effect of light intensity on the rate of photosynthesis. They punched 40 leaf disks from spinach leaves and used a syringe partially filled with water to pull the gases from the leaf disks so that all leaf disks sunk to the bottom of the syringe. Ten (10) leaf disks from the syringe were placed in each of four cups and covered with 50 ml of the solutions as indicated below. All leaf disks were resting on the bottom of the cups when the experiment began. The volume of liquid in each cup and the temperature of the solutions were held constant. All cups were placed 0.5 meters from the designated light source. A large beaker of water was placed between the light and the cups to act as a heat sink to prevent a change in temperature. At the end of 10 minutes, the number of disks floating in each cup was recorded.
Trial
1
2
3
4
Grams of baking soda (CO22source)
0.5
0.5
0.5
0
Wattage of light bulb
25
50
75
75
Disks floating at 10 minutes
3
5
9
0
Use your knowledge of the mechanism of photosynthesis and the data presented in the chart to determine which of the statements below is a correct explanation for the student's data.
A. Cup 2 had the highest rate of photosynthesis because 5 disks were floating at the end of 10 minutes using a 50 watt light bulb.
B. Cup 1 had a low rate of photosynthesis because 0.5 grams of baking soda did not provide a sufficient amount of CO2.
C. Cup 3 had the same rate of photosynthesis as Cup 1 because they had the same ratio of disks floating to wattage of light.
D. Cup 4 had the slowest rate of photosynthesis because it had the least baking soda.
D. Cup 4 had the slowest rate of photosynthesis because it had the least baking soda.
Photosynthesis ceases when leaves wilt, mainly because _____.
A. The chlorophyll in wilting leaves is degraded
B. Flaccid mesophyll cells are incapable of photosynthesis
C. Stomata close, preventing carbon dioxide from entering the leaf
D. Accumulation of carbon dioxide in the leaf inhibits enzymes
C. Stomata close, preventing carbon dioxide from entering the leaf
Which electron carrier(s) function in the citric acid cycle?
A. NADH and FADH2
B. The electron transport chain
C. NAD+
D. Only ADP and ATP
A. NADH and FADH2
The advantage of light microscopy over electron microscopy is that _____.
A. Light microscopy provides for higher resolving power than electron microscopy
B. Light microscopy provides higher contrast than electron microscopy
C. Light microscopy provides for higher magnification than electron microscopy
D. Light microscopy allows one to view dynamic processes in living cells
D. Light microscopy allows one to view dynamic processes in living cells