What is the molecular mass of caffeine (C8H10N4O2)?
a. 178.2
b. 43.0
c. 194.2
d. 138.2
e. None of the above.
c. 194.2
How many grams of water (H2O) are needed to react with 5.0 grams of ferric trichloride (FeCl3)?
FeCl3 + 3 H2O → Fe(OH)3 + 3 HCl
a. 1.7 g H2O
b. 0.99 g H2O
c. 0.18 g H2O
d. 0.55 g H2O
e. None of the above.
a. 1.7 g H2O
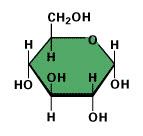
How many grams of glucose would you need to make 1 liter of an aqueous 0.5M glucose solution? (Atomic masses: C = 12.01, H = 1.008, O = 16.00.)
a. 90.08 grams of glucose
b. 120.1 grams of glucose
c. 180.2 grams of glucose
d. 70.36 grams of glucose
e. 60.05 grams of glucose
a. 90.08 grams of glucose
Undersea deposits that contain metals such as copper, gold, and silver are collectively known as what?
a. Massive phosphates
b. Massive sulfides
c. Massive chlorides
d. Massive oxides
b. Massive sulfides
The discovery of which of the following led to a drastic change in deep sea mining?
a. Royal gushers
b. Flat cyclers
c. Hot winders
d. Black smokers
d. Black smokers
You are an explorer in search of deep sea metal deposits. Which of the following would likely be the best place to look?
a. Near coral reefs
b. Along coastlines
c. In underwater caves
d. Near volcanic fissures
d. Near volcanic fissures
Which of the following is true?
a. The ores found in seabeds often have substantially higher purities than those found on land.
b. The ores found in seabeds usually have the same purities as those found on land.
c. The purities of ores found in seabeds cannot be compared to the purities of those found on land.
d. The ores found in seabeds often have substantially lower purities than those found on land.
a. The ores found in seabeds often have substantially higher purities than those found on land.
Your mining company wishes to export its metals to the country that consumes the most copper and gold. Where are you looking to send your products?
a. India
b. Russia
c. Mexico
d. China
d. China
The mass number of an atom is 15, and its atomic number is 7. The atom probably has...
a. 8 neutrons in the nucleus.
b. 7 electrons in the nucleus.
c. About as much mass in electrons as in protons.
d. At least 15 electrons.
e. 7 units of negative charge in the nucleus.
a. 8 neutrons in the nucleus.
Which statement is true of atoms?
a. Protons attract other protons.
b. Protons repel electrons.
c. Electrons determine the atom's size.
d. Most of an atom's volume is filled with matter.
e. All of the above.
c. Electrons determine the atom's size.
Dr. Jones says an atom has 3 electrons in the first shell and four electrons in the second shell. Someone should tell Dr. Jones that ...
a. No shell can hold more than 2 electrons.
b. The first shell must fill before the second shell can have electrons.
c. The first shell shouldn't have 3 electrons.
d. The second shell should have 8 electrons.
e. The second shell can't have 4 electrons.
c. The first shell shouldn't have 3 electrons.
Which statement is true of the energy levels of electrons in shells?
a. The valence shell has higher energy than other occupied shells.
b .Electrons must lose energy to move from the first to the second shell.
c. All the electrons in an atom have similar amounts of energy.
d. All of the above.
e. None of the above.
a. The valence shell has higher energy than other occupied shells.
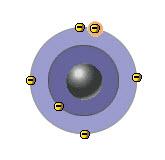
Which statement is true of the atom shown in the diagram?
a. The atom has more than one valence electron.
b. An electron will move from the outer to the inner shell.
c. The atom is in the excited state.
d. All of the above.
e. None of the above.
d. All of the above.
An orbital is dumbbell-shaped. Which statement is most likely true?
a. Its electrons move along a figure-8 path.
b. It's in the first electron shell.
b. Each lobe can hold one electron.
d. Each lobe can hold two electrons.
e. None of the above.
e. None of the above.
Two atoms always represent the same element if they have ...
a. The same mass number.
b. The same number of shells.
c. The same number of particles in the nucleus.
d. The same number of protons.
e. The same number of electrons.
d. The same number of protons.
An atom has 6 electrons, 6 protons, and 6 neutrons. You can tell that this atom belongs to the element _____ because _____________________.
a. O; its mass number is 12.
b. C; it has 6 electrons.
c. N; it has 6 protons.
d. N; it has 6 electrons.
e. C; it has 6 protons.
e. C; it has 6 protons.
An atom has 8 protons, 8 neutrons, and 8 electrons. Another isotope of the same element might have ...
a. 9 protons.
b. Mass number 16, atomic number 7.
c. 7 electrons.
d. 10 neutrons.
e. All of the above.
b. Mass number 16, atomic number 7.
Radioactive decay is likely to occur when ...
a. An atom has too many electrons.
b. An atom has too many neutrons.
c. Protons break into neutrons and electrons.
d. An electron hits the nucleus.
e. Atoms collide with one another.
b. An atom has too many neutrons.

Which model most accurately represents the current view of the structure of the atom?
a. Probability model
b. Planetary model
a. Probability model
Which statement is most useful in explaining why chemists assign atoms to chemical elements by counting protons?
a. Protons at the atom's surface determine the atom's behavior.
b. The proton's negative charge holds electrons in the atom.
c. The nucleus doesn't change in stable isotopes.
d. 99% of the atom's mass consists of protons.
e. None of these. Elements are defined by the number of protons.
e. None of these. Elements are defined by the number of protons.