Which of the following steps would NOT lead to variation of genetic material?
A. crossing over of homologous chromosomes
B. crossing over of sister chromatids
C. the random alignment of the chromosomes during metaphase I
D. the combination of sperm and egg genes
B
Which of the following is NOT a difference between anaphase I and anaphase II?
A. At the end of anaphase I, each chromosome is composed of two chromatids and at the end of anaphase II, sister chromatids have separated, becoming daughter chromosomes
B. Anaphase I occurs in a haploid cell while anaphase II occurs in a diploid cell.
C. Sister chromatids separate during anaphase II while homologous chromosomes separate during anaphase I.
D. The cell undergoing anaphase II is genetically different from what it contained while undergoing anaphase I.
B
The cell formed through fertilization of an egg by a sperm is called a/an
A. gamete.
B. sperm cell.
C. zygote.
D. egg cell.
E. ovum.
C
Determining the number of sperm in the individual
If a sperm cell contains 8 chromosomes, it comes from an animal that has ______ chromosomes.
A. 4
B. 8
C. 12
D. 16
E. 24
D
If a cell contains 12 chromosomes at the end of meiosis I, how many chromosomes will the daughter cells contain at the end of meiosis II?
A. 3
B. 6
C. 12
D. 24
C
During which stage of meiosis does crossing-over occur?
A. prophase I of meiosis I
B. anaphase I of meiosis II
C. telophase I of meiosis I
D. prophase II of meiosis II
E. anaphase II of meiosis I
A
What is the importance of crossing-over?
A. It provides extra genetic material for the daughter cells.
B. It increases the likelihood that daughter cells contain different genetic material.
C. It produces the proteins that are associated with DNA in chromosomes.
D. It increases chromosome condensation.
E. It separates the homologous chromosomes.
B
At which stage of meiosis is each chromosome composed of a single chromatid?
A. prophase I
B. metaphase II
C. anaphase II
D. prophase II
E. metaphase I
C
The genus Lacerta is composed of a species of lizards that are female and do not mate. They undergo "endomitosis" where one extra chromosome replication results in a tetraploid cell before meiosis begins. Normal female 2n offspring result without fertilization. What change(s) from regular meiosis (in preparation for fertilization) would be required to produce this system?
A. The haploid cell products of meiosis II fuse.
B. Meiosis stops after meiosis I and does not proceed to meiosis II.
C. Cytokinesis does not follow "endomitosis" that results in a tetraploid cell.
D. During anaphase II, the sister centromeres fail to separate and daughter cells will not form.
E. During anaphase II, the daughter chromosomes are non-disjunctive and are all pulled to one daughter cell.
C
Interkinesis is different from interphase in which way?
A. Interkinesis occurs after a cell finishes a nuclear division.
B. Interkinesis is the stage that precedes a prophase stage.
C. Interphase involves DNA replication and interkinesis does not.
D Interkinesis can be variable in length.
C
In human females, when is meiosis II completed?
A. at ovulation
B. immediately after the sperm penetration of the secondary oocyte
C. immediately after the sperm penetrates the primary oocyte
D. None of the choices are correct.
B
Where in the human male does spermatogenesis occur?
A. ovaries
B. prostate gland
C. epididymus
D. testes
D
During _______________ the homologous chromosome pairs separate in a random fashion leading to genetic diversity among the offspring.
A. independent assortment
B. metaphase
C. anaphase II
D. mitosis
A
In a classic Mendelian monohybrid cross between a homozygous dominant parent and a homozygous recessive parent, which generation is always completely heterozygous?
A. F1 generation
B. F2 generation
C. F3 generation
D. P generation
A
If a pea plant shows a recessive phenotype,
A. the genotype may be TT or Tt.
B. the genotype may be Tt or tt.
C. the genotype can only be TT.
D. the genotype can only be tt.
E. the genotype may be TT, Tt, or tt.
D
Women with X-linked disorders always pass the genes for the disorder to ______, while men with X-linked disorders always pass the genes for the disorder to _______.
A. only their daughters; only their daughters
B. both their daughters and sons; only their sons
C. both their daughters and sons; only their daughters
D. both their daughters and sons; their daughters and sons
C
What are alleles?
A. genes for different traits, such as hair color or eye color
B. alternative forms of a gene for a single trait, such as blue eyes or brown eyes
C. the locations of genes on a chromosome
D. recessive forms of a kind of characteristic carried by genes
E. dominant forms of a kind of characteristic carried by genes
B
If the parents are AO and BO genotypes for the ABO blood group, their children could include which of the following genotypes?
A. AO and BO only
B. AO, BO, and AB only
C. AA, BB, and AB only
D. AO, BO, and OO only
E. AO, BO, AB, and OO only
E
Unattached earlobes (EE or Ee) are described in the textbook as dominant over attached earlobes (ee). A couple both have unattached earlobes. Both notice that one of their parents on both sides has attached earlobes (ee). Therefore, they correctly assume that they are carriers for attached earlobes (Ee). The couple proceeds to have four children.
A. They can be certain that three will be heterozygous and one homozygous recessive.
B. If the first three are heterozygous, the fourth must be homozygous recessive.
C. The children must repeat the grandparents' genotype (Ee).
D. All children must have unattached earlobes since both parents possess the dominant gene for it.
E. Two heterozygous, one homozygous recessive and one homozygous dominant is a likely outcome, but all heterozygous, or two, three or all four homozygous are also possible.
E
A testcross involves an individual exhibiting the dominant phenotype but an unknown genotype being crossed with an individual that has a(n) ___________ genotype.
A. homozygous dominant
B. heterozygous dominant
C. homozygous recessive
D. any of the choices
C
If a human who is a tongue roller (T) and has unattached ear lobes (E) marries a person who cannot roll their tongue and has attached earlobes, could they produce an offspring that was also a non-tongue roller with attached earlobes? What would be the genotype of the first parent? the second parent?
A. yes; TtEe; tree
B. yes; TtEE; tree
C. no; TTEE; ttee
D. unable to determine from the information given
A
If black fur is produced by a recessive allele, which genotype is most likely to produce a black individual?
A. bb
B. BB
C. Bb
D. Cb
A
Transformation of bacteria was shown to occur when _______ bacteria were injected into mice and the mice _______.
A. live S strain; died
B. live R strain; didn't die
C. live S and R strain; died
D. live R strain and dead S strain; died
E. live S strain and dead R strain; didn't die
D
DNA was shown to be the transforming substance when only the ______ enzymes could inhibit transformation.
A. proteinase
B. RNAase
C. DNAase
D. lipase
C
If a DNA sample contains 13% adenine, what percentage of the sample contains cytosine?
A. 13%
B. 37%
C. 26%
D. 74%
B
The X-ray diffraction photography of Rosalind Franklin and Maurice Wilkins was critical evidence in the study of DNA,
A. indicating that DNA has a double helix structure.
B. showing equal numbers of purines and pyrimidines.
C. showing the bases of DNA were held together by hydrogen bonds.
D. revealing the structure of the deoxyribose sugar.
E. of the location of each adenine, guanine, cytosine, and thymine.
A
In the Watson and Crick model of DNA, the "steps" of the ladder are composed of
A. sugars.
B. a purine and a pyrimidine.
C. two purines.
D. two pyrimidines.
E. a sugar and a phosphate molecule.
B
Nucleotides contain all of the following except:
A. a phosphate group
B. a 5 - carbon sugar
C. a nitrogen base
D. histones
E. a phosphate and a nitrogen base
D
During DNA replication, the enzyme ___________, catalyzes the elongation of new DNA by adding, to the 3' end of the previous nucleotide, new nucleotides that are complementary to a DNA template.
A. helicase
B. DNA polymerase
C. DNA ligase
D. ATP synthase
B
Which statement is NOT true about DNA replication?
A. It proceeds in a 5'-to-3' direction only.
B. One strand of new DNA is replicated faster than the other strand at the replication fork.
C. DNA can only replicate at one point on a chromosome at one time.
D. It occurs more rapidly in bacteria than in eukaryotes.
E. Replication can only begin at a special origin of replication.
C
Which statement is NOT true about DNA replication in prokaryotes?
A. Replication begins at a single origin of replication.
B. Replication is bidirectional from the origin(s).
C. Replication occurs at about 1 million base pairs per minute.
D. Since bacterial cells replicate so rapidly, a second round of replication may begin before the first has been completed.
E. There are many bacterial chromosomes, with replication occurring in each at the same time.
E
Which statement is NOT true about DNA replication in eukaryotes?
A. Replication of the entire genome takes about ten minutes.
B. A replication fork occurs at each growing point of the replicating chromosome(s).
C. Eukaryotes have many different chromosomes, with replication occurring in each at the same time.
D. Replication occurs at the rate of about 500-5000 base pairs per minute.
E. Multiple sites of replication are present on each chromosome.
A
DNA replication is considered semiconservative because:
A. it will create three new, identical strands when finished.
B. it uses the original strand as a template for replication.
C. it always replicates in the 3 to 5 prime direction.
D. it never replicates in the 5 to 3 prime direction.
B
Which is NOT true about the genetic code?
A. Most amino acids have only one codon.
B. It is composed of a triplet code of three bases per codon.
C. It produces 64 different possibilities of base sequences.
D. It was cracked through the use of a cell-free system of enzymes.
E. It contains start and stop codons as instructions.
A
The correct sequence of events in translation is:
A. initiation, termination, elongation.
B. elongation, termination, initiation.
C. termination, elongation, initiation.
D. elongation, initiation, termination.
E. initiation, elongation, termination.
E
An unknown chemical is analyzed and found to contain the bases thymine and guanine. This chemical is most likely
A. tRNA.
B. mRNA.
C. DNA.
D. rRNA.
C
Transcription of a part of a DNA molecule with a nucleotide sequence of A-A-A-C-A-A-C-T-T results in a mRNA molecule with the complementary sequence of
A. G-G-G-A-G-A-A-C-C.
B. U-U-U-C-U-U-C-A-A
C. U-U-U-G-U-U-G-A-A.
D. T-T-T-G-A-A-G-C-C.
E. C-C-C-A-C-C-T-C-C.
F. none of the choices are correct.
C
If one strand of DNA has the base sequence AAGCAA, the complementary strand has which of the following sequences?
A. UUCGUU
B. TTCGTT
C. AAGCAA
D. UTCGTU
E. TTCGTG
B
Transcription is initiated when:
A. initiation factors assemble ribosomal subunits, mRNA, and initiator tRNA.
B. RNA polymerase comes to a stop sequence.
C. RNA polymerase binds to a region of DNA called the promoter.
D. new nucleotides are added to an existing strand of nucleotides.
C
During the elongation of a polypeptide chain, _________ occurs when the mRNA moves to the next site on the ribosome to read the next codon.
A. translocation
B. transcription
C. translation
D. transference
A
Which of the following would be transcribed into mRNA?
A. a noncoding gene
B. a protein-coding gene
C. a promoter
D. a ribozyme
B
A (an) _______ is a group of three bases on tRNA that is complementary to a specific mRNA codon.
A. codon
B. anticodon
C. poly-A tail
D. cap
B
In the lac operon, if lactose is present, which of the following occurs?
A. Lactose binds to the repressor, changing its shape so that it can bind to the operator and the structural genes are not expressed.
B. Lactose bind to RNA polymerase, which then binds to the promoter and transcribes the needed genes.
C. Lactose binds to the repressor, changing its shape so that it does not bind to the operator. RNA polymerase then transcribes the needed genes.
D. Lactose binds to the operon, which attracts RNA polymerase, then transcription of the needed genes occurs.
C
Active genes in eukaryotic cells are associated with
A. euchromatin.
B. heterochromatin.
C. methylated RNA and histones.
D. DNA with many methyl groups.
A
Which component in an operon is incorrectly matched with its function?
A. promoter—where RNA polymerase first binds to DNA
B. regulator gene—binds to the repressor protein
C. structural gene—makes mRNA by transcription
D. operator—if not bound to repressor, allows RNA polymerase to bind to DNA
B
Which statement is NOT correct about the lac operon?
A. It regulates the production of a series of five enzymes.
B. It is normally turned off if glucose is present.
C. Lactose binds to the repressor protein and inactivates it.
D. It is an inducible system.
E. The structural genes make products that allow lactose metabolism.
A
Which statement is NOT correct about the trp operon?
A. The structural genes make products that are part of an anabolic pathway for the synthesis of tryptophan.
B. It is normally turned off if tryptophan is present.
C. Tryptophan acts as the corepressor.
D. The regulator gene product is inactive by itself.
E. Tryptophan binds to the repressor protein and inactivates it.
E
_______ control is the most critical level of eukaryotic genetic control.
A. Feedback
B. Translational
C. Transcriptional
D. Posttranscriptional
E. Posttranslational
C
_________ control is the level of genetic control that involves the life span of the mRNA molecule and the ability of the mRNA to bind to ribosomes.
A. Feedback control
B. Translational control
C. Transcriptional control
D. Posttranscriptional control
E. Posttranslational control
B
_________ is the level of genetic control that involves the processing of early RNA transcripts to mRNA and the rate at which mRNA leaves the nucleus.
A. Feedback control
B. Translational control
C. Transcriptional control
D. Posttranscriptional control
E. Posttranslational control
D
Which level of primary control in eukaryotic gene activity involves changes in the polypeptide chain before it becomes functional?
A. feedback control
B. translational control
C. transcriptional control
D. posttranscriptional control
E. posttranslational control
E
A form of gene regulation that occurs while RNA is still in the nucleus is
A. differential intron removal and splicing.
B. feedback control.
C. enzymatic cleavage of a polypeptide.
D. rate of binding to ribosomes.
A
You are more likely to develop some forms of cancer if you
A. are exposed to higher doses of radiation including X rays.
B. are exposed to carcinogens.
C. have a high incidence of cancer in your family history.
D. All of the choices are correct.
D
The DNA of a _______ is wrapped around histone molecules to form a "beaded string."
A. prokaryote
B. eukaryote
C. bacterium
D. All of the above.
B
Androgen insensitivity is characterized by
A. a lack of male hormones, such as testosterone.
B. external female genitalia.
C. a female karyotype.
D. the internal sex organs of a female and may bear children.
B
If a chromosome is highly methylated,
A. the gene is expressed, producing more gene product than normal.
B. the gene is not expressed, even if it is a normal gene in every other way.
C. the gene is expressed, but reduced quantities protein are made.
D. there is no affect on gene expression.
B
What is the function of naturally occurring restriction enzymes in bacterial cells?
A. They are used during DNA replication in the bacterial cell.
B. They are used to degrade the bacterial cell's DNA.
C. Restriction enzymes recognize and cleave DNA molecules that are foreign to the bacterial cell.
D. These enzymes are used to attach pieces of DNA into an opening created by ligase enzymes.
C
Which of the following is mismatched?
A. bioinformatics - the study of a genomic and proteomic information using computer analysis
B. polymerase chain reaction - process that separates DNA fragments according to size
C. genomics - the study of the genomes of humans and other organisms
D. proteomics - the study of species' proteins
B
Which of the following molecules forms lengths of DNA with "sticky ends"?
A. DNA ligase
B. DNA polymerase
C. RNA polymerase
D. reverse transcriptase
E. restriction enzyme
E
The function of DNA ligase in recombinant technology is to
A. cut DNA into many fragments.
B. carry DNA into a new cell.
C. seal DNA into an opening created by restriction enzymes.
D. make millions of copies of a specific segment of DNA.
E. separate fragments of DNA by their length and electrical charges.
C
All of the following statements are true about restriction enzymes EXCEPT
A. they are made by bacteria and viruses.
B. hundreds of different ones have been isolated and purified.
C. they produce single-stranded complementary ends that can join to two different DNA strands by complementary base pairing.
D. each enzyme cuts DNA at a different specific base sequence.
A
Plants are being genetically engineered to have
A. a requirement for more fertilizer.
B. an increased water requirement.
C. the ability to produce human proteins.
D. increased susceptibility to herbicides.
E All of the choices are correct
C
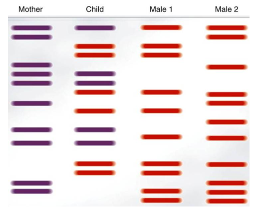
DNA fingerprinting may be used to establish paternity. Analyze the banding pattern to determine the father of the child.
A. Male 1
B. Male 2
A
Which of the following is the correct order of steps in cloning a human gene?
A. DNA ligase seals the human gene and plasmid.
B. Host cell takes up the plasmid.
C. Gene cloning takes place as the cell undergoes the cell cycle.
D. Restriction enzyme is used to cleave the DNA.
A. D-A-B-C
B. A-C-D-B
C. D-A-C-B
D. D-B-C-A
A
A localized group of organisms that belong to the same species is called a
A) biosystem
B) community
C) population
D) ecosystem
E) family
C
Once labor begins in childbirth, contractions increase in intensity and frequency until delivery. The increasing labor contractions of childbirth are an example of which type of regulation?
A) a bioinformatic system
B) positive feedback
C) negative feedback
D) feedback inhibition
E) enzymatic catalysis
B
When the body's blood glucose level rises, the pancreas secretes insulin and, as a result, the blood glucose level declines. When the blood glucose level is low, the pancreas secretes glucagon and, as a result, the blood glucose level rises. Such regulation of the blood glucose level is the result of
A) catalytic feedback
B) positive feedback
C) negative feedback
D) bioinformatic regulation
E) protein-protein interactions
C
Which branch of biology is concerned with the naming and classifying of organisms?
A) informatics
B) schematic biology
C) taxonomy
D) genomics
E) evolution
C
Prokaryotes are classified as belonging to two different domains. What are the domains?
A) Bacteria and Eukarya
B) Archaea and Monera
C) Eukarya and Monera
D) Bacteria and Protista
E) Bacteria and Archaea
E
Global warming, as demonstrated by observations such as melting of glaciers, increasing CO2 levels, and increasing average ambient temperatures, has already had many effects on living organisms. Which of the following might best offer a solution to this problem?
A) Continue to measure these and other parameters of the problem.
B) Increase the abilities of animals to migrate to more suitable habitats.
C) Do nothing; nature will attain its own balance.
D) Limit the burning of fossil fuels and regulate our loss of forested areas.
E) Recycle as much as possible.
D
A water sample from a hot thermal vent contained a single-celled organism that had a cell wall but lacked a nucleus. What is its most likely classification?
A) Eukarya
B) Archaea
C) Animalia
D) Protista
E) Fungi
B
A controlled experiment is one in which
A) the experiment is repeated many times to ensure that the results are accurate.
B) the experiment proceeds at a slow pace to guarantee that the scientist can carefully observe all reactions and process all experimental data.
C) there are at least two groups, one of which does not receive the experimental treatment.
D) there are at least two groups, one differing from the other by two or more variables.
E) there is one group for which the scientist controls all variables.
C
Why is it important that an experiment include a control group?
A) The control group is the group that the researcher is in control of, the group in which the researcher predetermines the results.
B) The control group provides a reserve of experimental subjects.
C) A control group is required for the development of an "If…then" statement.
D) A control group assures that an experiment will be repeatable.
E) Without a control group, there is no basis for knowing if a particular result is due to the variable being tested.
E
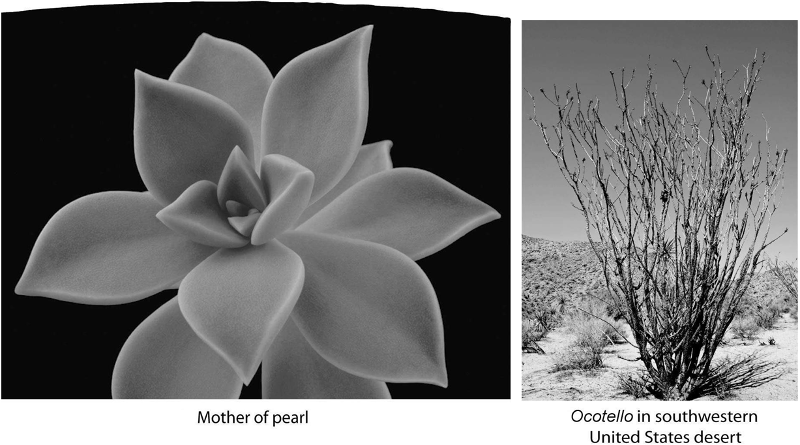
What do these two plants have in common?
A) adaptations to extreme heat
B) adaptations to conserve water
C) identical stem structures
D) identical flower structures
E) lack of photosynthesis
B
All the organisms on your campus make up
A) an ecosystem.
B) a community.
C) a population.
D) an experimental group.
E) a taxonomic domain.
B
Why is each element unique and different from other elements in chemical properties?
A) Each element has a unique atomic mass.
B) Each element has a unique atomic weight.
C) Each element has a unique number of protons in its nucleus.
D) Each element has a unique number of neutrons in its nucleus.
E) Each element has different radioactive properties.
C
Knowing just the atomic mass of an element allows inferences about which of the following?
A) the chemical properties of the element
B) the number of protons in the element
C) the number of neutrons in the element
D) the number of protons plus neutrons in the element
E) both the number of protons and the chemical properties of the element
D
Molybdenum has an atomic number of 42. Several common isotopes exist, with mass numbers of 92, 94, 95, 96, 97, 98, and 100. Therefore, which of the following can be true?
A) Molybdenum atoms can have between 50 and 58 neutrons.
B) The isotopes of molybdenum have different electron configurations.
C) The isotopes of molybdenum can have between 50 and 58 protons.
D) The isotopes of molybdenum have between 50 and 58 neutrons and have different electron configurations.
E) The isotopes of molybdenum have between 50 and 58 protons and have different electron configurations.
A
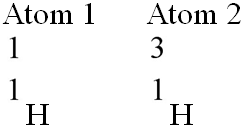
Which of the following best describes the relationship between the atoms described below? [SEE IMAGE]
A) They are isomers.
B) They are polymers.
C) They are isotopes.
D) They contain 1 and 3 protons, respectively.
E) They each contain 1 neutron.
C
One difference between carbon-12 (12/6 C) is that carbon-14 (14/6 C) has
A) two more protons than carbon-12.
B) two more electrons than carbon-12.
C) two more neutrons than carbon-12.
D) two more protons and two more neutrons than carbon-12.
E) two more electrons and two more neutrons than carbon-12.
C
The atomic number of neon is 10. Therefore, which of the following is most correct about an atom of neon?
A) It has 8 electrons in its outer electron shell.
B) It is inert.
C) It has an atomic mass of 10 daltons.
D) It has 8 electrons in its outer electron shell and it is inert.
E) It has 8 electrons in its outer electron shell, it is inert, and it has an atomic mass of 10 daltons.
D
From its atomic number of 15, it is possible to predict that the phosphorus atom has
A) 15 neutrons.
B) 15 protons.
C) 15 electrons.
D) 8 electrons in its outermost electron shell.
E) 15 protons and 15 electrons.
E
The atomic number of each atom is given to the left of each of the elements below. Which of the atoms has the same valence as carbon (12/6 C)?
A) ₇N nitrogen
B) ₉F flourine
C) ₁₀Ne neon
D) ₁₂Mg magnesium
E) ₁₄Si silicon
E
An atom with atomic number 12 would have what type of chemical behavior in bonding with other elements?
A) It would form ions with a +1 charge.
B) It would form ions with a +2 charge.
C) It would form ions with a -1 charge.
D) It would form ions with a -2 charge.
E) It would form two covalent bonds with other atoms.
B
A covalent chemical bond is one in which
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms.
C) outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms.
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom.
E) an electron occupies a hybrid orbital located between the nuclei of two atoms.
C
When two atoms are equally electronegative, they will interact to form
A) hydrogen bonds.
B) van der Waals interactions.
C) polar covalent bonds.
D) nonpolar covalent bonds.
E) ionic bonds.
D
What results from an unequal sharing of electrons between atoms?
A) a nonpolar covalent bond
B) a polar covalent bond
C) an ionic bond
D) a hydrogen bond
E) a hydrophobic interaction
B
Which of the following molecules contains the most polar covalent bond?
A) H₂
B) O₂
C) CO₂
D) H₂O
E) CH₄
D
What is the difference between covalent bonds and ionic bonds?
A) Covalent bonds are formed between atoms to form molecules; ionic bonds are formed between atoms to form compounds.
B) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between atoms.
D) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between atoms.
E) Covalent bonds involve the transfer of electrons between atoms; ionic bonds involve the sharing of electrons between atoms.
C
Van der Waals interactions result when
A) hybrid orbitals overlap.
B) electrons are not symmetrically distributed in a molecule.
C) molecules held by ionic bonds react with water.
D) two polar covalent bonds react.
E) a hydrogen atom loses an electron.
B
Which of the following is not considered to be a weak molecular interaction?
A) a covalent bond
B) a van der Waals interaction
C) an ionic bond in the presence of water
D) a hydrogen bond
E) both a hydrogen bond and a covalent bond
A

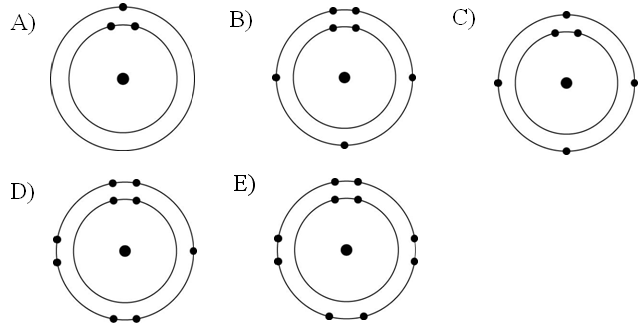
In the figure above, how many electrons does nitrogen have in its valence shell?
A) 2
B) 5
C) 7
D) 8
E) 14
B

In the figure above, how many unpaired electrons does phosphorus have in its valence shell?
A) 15
B) 2
C) 3
D) 7
E) 5
C

How many neutrons are present in the nucleus of a phosphorus-32 (³²P) atom (see the figure above)?
A) 5
B) 15
C) 16
D) 17
E) 32
D

How many electrons does an atom of sulfur have in its valence shell (see the figure above)?
A) 4
B) 6
C) 8
D) 16
E) 32
B
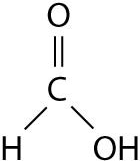
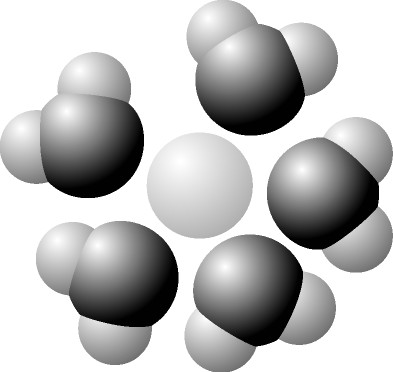
The illustration above shows a representation of formic acid. A formic acid molecule
A) will form hydrogen bonds with water molecules.
B) has a tetrahedral configuration of hybrid electron orbitals for the carbon atom.
C) consists of largely nonpolar covalent bonds.
D) is held together by hydrogen bonds.
E) has a tetrahedral shape and will form hydrogen bonds with water molecules.
A
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A
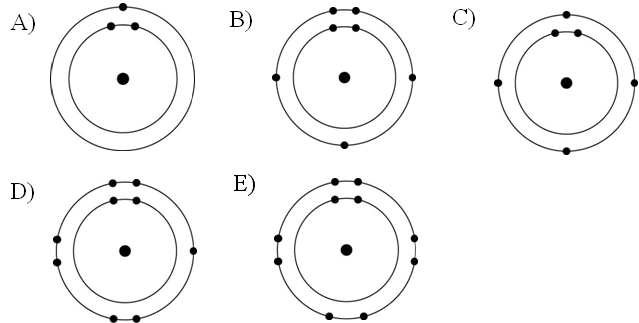
Which one of the atoms shown would be most likely to form an anion with a charge of -1?
D
A group of molecular biologists is trying to synthesize a new artificial compound to mimic the effects of a known hormone that influences sexual behavior. They have turned to you for advice. Which of the following compounds is most likely to mimic the effects of the hormone?
A) a compound with the same number of carbon atoms as the hormone
B) a compound with the same molecular mass (measured in daltons) as the hormone
C) a compound with the same three-dimensional shape as part of the hormone
D) a compound with the same number of orbital electrons as the hormone
E) a compound with the same number of hydrogen and nitrogen atoms as the hormone
C
Which of the following effects is produced by the high surface tension of water?
A) Lakes don't freeze solid in winter, despite low temperatures.
B) A water strider can walk across the surface of a small pond.
C) Organisms resist temperature changes, although they give off heat due to chemical reactions.
D) Evaporation of sweat from the skin helps to keep people from overheating.
E) Water flows upward from the roots to the leaves in plants.
B
Liquid water's high specific heat is mainly a consequence of the
A) small size of the water molecules.
B) high specific heat of oxygen and hydrogen atoms.
C) absorption and release of heat when hydrogen bonds break and form.
D) fact that water is a poor heat conductor.
E) higher density of liquid water than solid water (ice).
C
Which type of bond must be broken for water to vaporize?
A) ionic bonds
B) both hydrogen bonds and ionic bonds
C) polar covalent bonds
D) hydrogen bonds
E) both polar covalent bonds and hydrogen bonds
D
Why does evaporation of water from a surface cause cooling of the surface?
A) The breaking of bonds between water molecules absorbs heat.
B) The water molecules with the most heat energy evaporate more readily.
C) The solute molecules left behind absorb heat.
D) Water molecules absorb heat from the surface in order to acquire enough energy to evaporate.
E) The expansion of water vapor extracts heat from the surface.
B
Hydrophobic substances such as vegetable oil are
A) nonpolar substances that repel water molecules.
B) nonpolar substances that have an attraction for water molecules.
C) polar substances that repel water molecules.
D) polar substances that have an affinity for water.
E) charged molecules that hydrogen-bond with water molecules.
A
Which of the following solutions would require the greatest amount of base to be added to bring the solution to neutral pH?
A) gastric juice at pH 2
B) vinegar at pH 3
C) tomato juice at pH 4
D) black coffee at pH 5
E) household bleach at pH 12
A
Which of the following statements is true about buffer solutions?
A) They maintain a constant pH when bases are added to them but not when acids are added to them.
B) They maintain a constant pH when acids are added to them but not when bases are added to them.
C) They maintain a relatively constant pH of approximately 7 when either acids or bases are added to them.
D) They maintain a relatively constant pH when either acids or bases are added to them.
E) They are found only in living systems and biological fluids.
D
Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely
A) positively charged.
B) negatively charged.
C) without charge.
D) hydrophobic.
E) nonpolar.
A
Which of these molecules would be soluble in water?
B
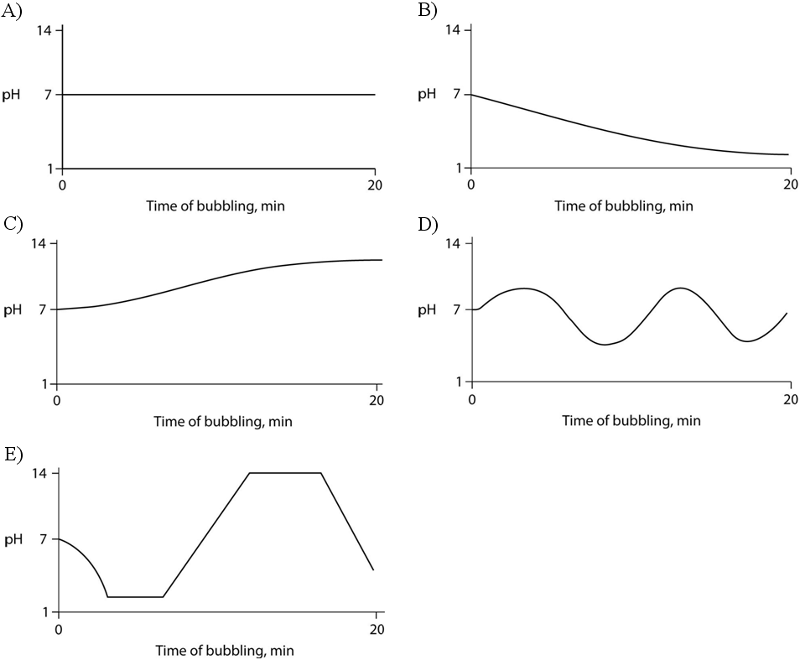
Carbon dioxide (CO₂) is readily soluble in water, according to the equation CO₂ + H₂O ↔ H₂CO₃. Carbonic acid (H₂CO₃) is a weak acid. If CO₂ is bubbled into a beaker containing pure, freshly distilled water, which of the following graphs correctly describes the results?
B
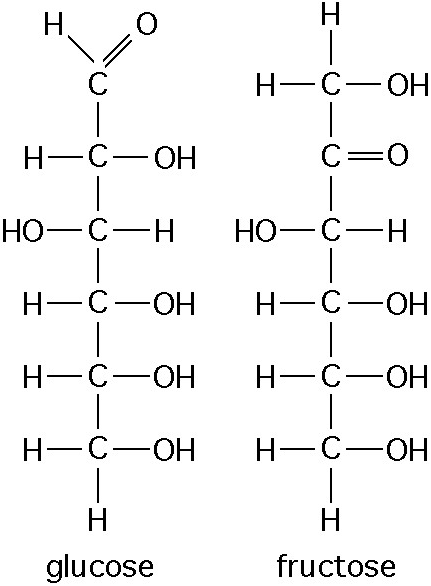
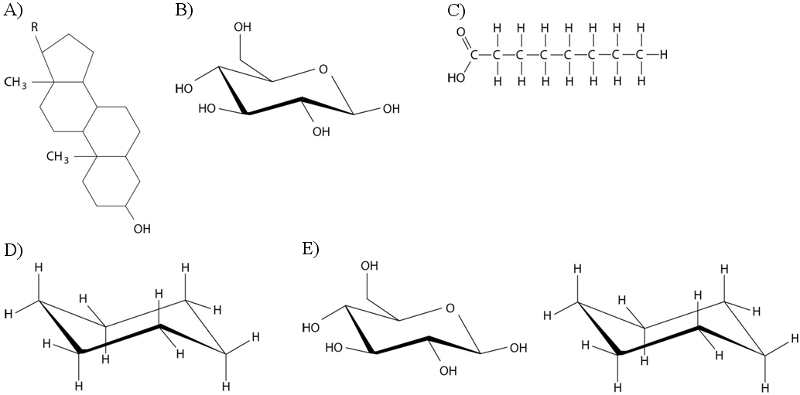
The figure above shows the structures of glucose and fructose. These two molecules differ in the
A) number of carbon, hydrogen, and oxygen atoms.
B) types of carbon, hydrogen, and oxygen atoms.
C) arrangement of carbon, hydrogen, and oxygen atoms.
D) number of oxygen atoms joined to carbon atoms by double covalent bonds.
E) number of carbon, hydrogen, and oxygen atoms; the types of carbon, hydrogen, and oxygen atoms; and the arrangement of carbon, hydrogen, and oxygen atoms.
C
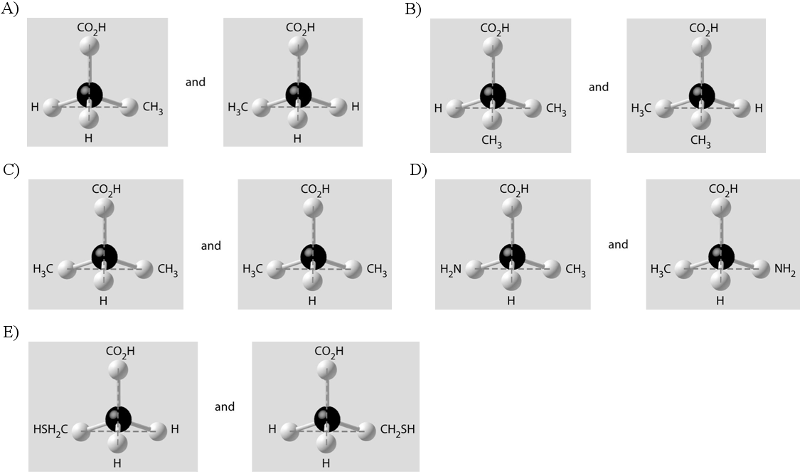
Which of the pairs of molecular structures shown below depict enantiomers (enantiomeric forms) of the same molecule?
D
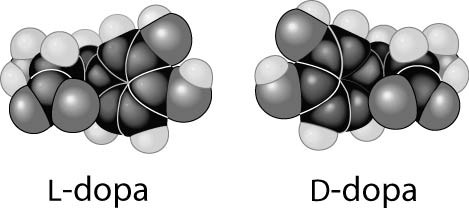
Thalidomide and L-dopa, shown below, are examples of pharmaceutical drugs that occur as enantiomers, or molecules that
A) have identical three-dimensional shapes.
B) are mirror images of one another.
C) are structural isomers.
D) are mirror images of one another and have the same biological activity.
E) are cis-trans isomers.
B
Which of these molecules is not formed by dehydration reactions?
A) fatty acids
B) disaccharides
C) DNA
D) protein
E) amylose
A
Which of the following is not a polymer?
A) glucose
B) starch
C) cellulose
D) chitin
E) DNA
A
What is the chemical reaction mechanism by which cells make polymers from monomers?
A) phosphodiester linkages
B) hydrolysis
C) dehydration reactions
D) ionic bonding of monomers
E) the formation of disulfide bridges between monomers
C
How many molecules of water are needed to completely hydrolyze a polymer that is 11 monomers long?
A) 12
B) 11
C) 10
D) 9
E) 8
C
Which of the following best summarizes the relationship between dehydration reactions and hydrolysis?
A) Dehydration reactions assemble polymers, and hydrolysis reactions break down polymers.
B) Dehydration reactions eliminate water from lipid membranes, and hydrolysis makes lipid membranes water permeable.
C) Dehydration reactions can occur only after hydrolysis.
D) Hydrolysis creates monomers, and dehydration reactions break down polymers.
E) Dehydration reactions ionize water molecules and add hydroxyl groups to polymers; hydrolysis reactions release hydroxyl groups from polymers.
A
On food packages, to what does the term insoluble fiber refer?
A) cellulose
B) polypeptides
C) starch
D) amylopectin
E) chitin
A
Humans can digest starch but not cellulose because
A) the monomer of starch is glucose, while the monomer of cellulose is galactose.
B) humans have enzymes that can hydrolyze the β glycosidic linkages of starch but not the α glycosidic linkages of cellulose.
C) humans have enzymes that can hydrolyze the α glycosidic linkages of starch but not the β glycosidic linkages of cellulose.
D) humans harbor starch-digesting bacteria in the digestive tract.
E) the monomer of starch is glucose, while the monomer of cellulose is glucose with a nitrogen-containing group.
C
Which of the following statements concerning saturated fats is not true?
A) They are more common in animals than in plants.
B) They have multiple double bonds in the carbon chains of their fatty acids.
C) They generally solidify at room temperature.
D) They contain more hydrogen than unsaturated fats having the same number of carbon atoms.
E) They are one of several factors that contribute to atherosclerosis.
B
A molecule with the formula C₁₈H3₆O₂ is probably a
A) carbohydrate.
B) fatty acid.
C) protein.
D) nucleic acid.
E) hydrocarbon.
B
The label on a container of margarine lists "hydrogenated vegetable oil" as the major ingredient. What is the result of adding hydrogens to vegetable oil?
A) The hydrogenated vegetable oil has a lower melting point.
B) The hydrogenated vegetable oil stays solid at room temperature.
C) The hydrogenated vegetable oil has more "kinks" in the fatty acid chains.
D) The hydrogenated vegetable oil has fewer trans fatty acids.
E) The hydrogenated vegetable oil is less likely to clog arteries.
B
Why are human sex hormones considered to be lipids?
A) They are essential components of cell membranes.
B) They are not soluble in water.
C) They are made of fatty acids.
D) They are hydrophilic compounds.
E) They contribute to atherosclerosis.
B
The bonding of two amino acid molecules to form a larger molecule requires
A) the release of a water molecule.
B) the release of a carbon dioxide molecule.
C) the addition of a nitrogen atom.
D) the addition of a water molecule.
E) the release of a nitrous oxide molecule.
A
There are 20 different amino acids. What makes one amino acid different from another?
A) different side chains (R groups) attached to a carboxyl carbon
B) different side chains (R groups) attached to the amino groups
C) different side chains (R groups) attached to an α carbon
D) different structural and optical isomers
E) different asymmetric carbons
C
Polysaccharides, triacylglycerides, and proteins are similar in that they
A) are synthesized from monomers by the process of hydrolysis.
B) are synthesized from subunits by dehydration reactions.
C) are synthesized as a result of peptide bond formation between monomers.
D) are decomposed into their subunits by dehydration reactions.
E) all contain nitrogen in their monomer building blocks.
B
Dehydration reactions are used in forming which of the following compounds?
A) triacylglycerides
B) polysaccharides
C) proteins
D) triacylglycerides and proteins only
E) triacylglycerides, polysaccharides, and proteins
E
Upon chemical analysis, a particular polypeptide was found to contain 100 amino acids. How many peptide bonds are present in this protein?
A) 101
B) 100
C) 99
D) 98
E) 97
C
What aspects of protein structure are stabilized or assisted by hydrogen bonds?
A) primary structure
B) secondary structure
C) tertiary structure
D) quaternary structure
E) secondary, tertiary, and quaternary structures, but not primary structure
E
Which bonds are created during the formation of the primary structure of a protein?
A) peptide bonds
B) hydrogen bonds
C) disulfide bonds
D) phosphodiester bonds
E) peptide bonds, hydrogen bonds, and disulfide bonds
A
Misfolding of polypeptides is a serious problem in cells. Which of the following diseases are associated with an accumulation of misfolded polypeptides?
A) Alzheimer's only
B) Parkinson's only
C) diabetes mellitus only
D) Alzheimer's and Parkinson's only
E) Alzheimer's, Parkinson's, and diabetes mellitus
D
What is the term used for a protein molecule that assists in the proper folding of other proteins?
A) tertiary protein
B) chaperonin
C) enzyme protein
D) renaturing protein
E) denaturing protein
B
Which of the following are nitrogenous bases of the purine type?
A) cytosine and guanine
B) guanine and adenine
C) adenine and thymine
D) thymine and uracil
E) uracil and cytosine
B
If a DNA sample were composed of 10% thymine, what would be the percentage of guanine?
A) 10
B) 20
C) 40
D) 80
E) impossible to tell from the information given
C
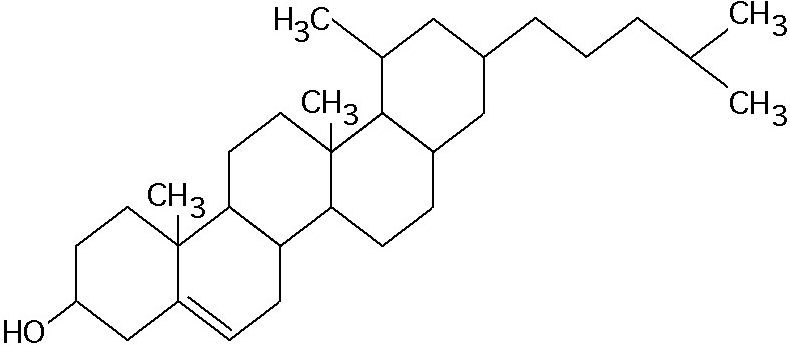
What is the structure shown in Figure 5.4?
A) pentose molecule
B) fatty acid molecule
C) steroid molecule
D) oligosaccharide molecule
E) phospholipid molecule
C
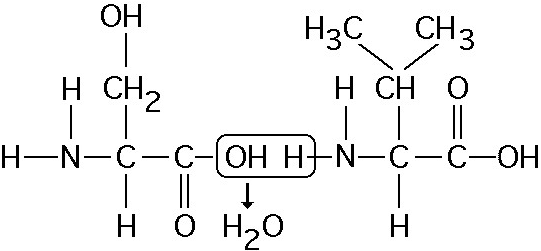
Which of the following statements is/are true regarding the chemical reaction illustrated in Figure 5.5?
A) It is a hydrolysis reaction.
B) It results in a peptide bond.
C) It joins two fatty acids together.
D) It is a hydrolysis reaction and it results in a peptide bond.
E) It is a hydrolysis reaction, it results in a peptide bond, and it joins two fatty acids together.
B
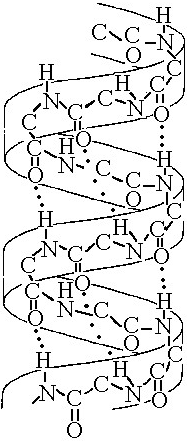
The structure depicted in Figure 5.7 shows the
A) 1-4 linkage of the α glucose monomers of starch.
B) 1-4 linkage of the β glucose monomers of cellulose.
C) double-helical structure of a DNA molecule.
D) α helix secondary structure of a polypeptide.
E) β pleated sheet secondary structure of a polypeptide.
D
Cyanide binds with at least one molecule involved in producing ATP. If a cell is exposed to cyanide, most of the cyanide will be found within the
A) mitochondria.
B) ribosomes.
C) peroxisomes.
D) lysosomes.
E) endoplasmic reticulum.
A
A biologist ground up some plant leaf cells and then centrifuged the mixture to fractionate the organelles. Organelles in one of the heavier fractions could produce ATP in the light, whereas organelles in the lighter fraction could produce ATP in the dark. The heavier and lighter fractions are most likely to contain, respectively,
A) mitochondria and chloroplasts.
B) chloroplasts and peroxisomes.
C) peroxisomes and chloroplasts.
D) chloroplasts and mitochondria.
E) mitochondria and peroxisomes.
D
ECM proteins are made by ribosomes in which part of a eukaryotic cell?
A) mitochondria
B) cytoplasm
C) nuclear envelope
D) Golgi apparatus
E) rough ER
E
A biologist ground up some plant leaf cells and then centrifuged the mixture to fractionate the organelles. Organelles in one of the heavier fractions could produce ATP in the light, whereas organelles in the lighter fraction could produce ATP in the dark. The heavier and lighter fractions are most likely to contain, respectively,
A) mitochondria and chloroplasts.
B) chloroplasts and peroxisomes.
C) peroxisomes and chloroplasts.
D) chloroplasts and mitochondria.
E) mitochondria and peroxisomes.
D
Recent evidence shows that signals from the extracellular matrix (ECM) can regulate the expression of genes in the cell nucleus. A likely mechanism is that
A) mechanical signals of the ECM can alter the cytoskeleton, which can alter intracellular signaling.
B) intracellular signals might cause changes in the fibronectin binding to the cell surface.
C) orientation of microtubules to the ECM can change gene activity.
D) integrins that receive signals from the ECM migrate to the nucleus.
E) proteoglycans in the ECM undergo endocytosis and produce intracellular signaling molecules.
A
Ions can travel directly from the cytoplasm of one animal cell to the cytoplasm of an adjacent cell through
A) plasmodesmata.
B) intermediate filaments.
C) tight junctions.
D) desmosomes.
E) gap junctions.
E
Plasmodesmata in plant cells are most similar in function to which of the following structures in animal cells?
A) peroxisomes
B) desmosomes
C) gap junctions
D) extracellular matrix
E) tight junctions
C
The cell walls of bacteria, fungi, and plant cells and the extracellular matrix of animal cells are all external to the plasma membrane. Which of the following is a characteristic common to all of these extracellular structures?
A) They must block water and small molecules in order to regulate the exchange of matter and energy with their environment.
B) They must permit information transfer between the cell's cytoplasm and the nucleus.
C) They must provide a rigid structure that maintains an appropriate ratio of cell surface area to volume.
D) They are constructed of polymers that are synthesized in the cytoplasm and then transported out of the cell.
E) They are composed of a mixture of lipids and carbohydrates.
D
All of the following serve an important role in determining or maintaining the structure of plant cells. Which of the following are distinct from the others in their composition?
A) microtubules
B) microfilaments
C) plant cell walls
D) intermediate filaments
E) nuclear lamina
C
Cytochalasin D is a drug that prevents actin polymerization. A cell treated with cytochalasin D will still be able to
A) perform amoeboid movement.
B) form cleavage furrows.
C) contract muscle fibers.
D) extend pseudopodia.
E) move vesicles around the cell.
E
Movement of vesicles within the cell depends on what cellular structures?
A) microtubules and motor proteins
B) actin filaments and microtubules
C) actin filaments and ribosomes
D) centrioles and motor proteins
E) actin filaments and motor proteins
A
Why isn't the mitochondrion classified as part of the endomembrane system?
A) It is a static structure.
B) Its structure is not derived from the ER or Golgi.
C) It has too many vesicles.
D) It is not involved in protein synthesis.
E) It is not attached to the outer nuclear envelope.
B
Which type of organelle is found in plant cells but not in animal cells?
A) ribosomes
B) mitochondria
C) nuclei
D) plastids
E) none of these
D
A cell has the following molecules and structures: enzymes, DNA, ribosomes, plasma membrane, and mitochondria. It could be a cell from
A) a bacterium.
B) an animal, but not a plant.
C) nearly any eukaryotic organism.
D) any multicellular organism, like a plant or an animal.
E) any kind of organism.
C
One of the key innovations in the evolution of eukaryotes from a prokaryotic ancestor is the endomembrane system. What eukaryotic organelles or features might have evolved as a part of, or as an elaboration of, the endomembrane system?
A) plasma membrane
B) chloroplasts
C) mitochondria
D) nuclear envelope
E) none of these
D
Thylakoids, DNA, and ribosomes are all components found in
A) vacuoles.
B) chloroplasts.
C) mitochondria.
D) lysosomes.
E) nuclei.
B
Which organelle is the primary site of ATP synthesis in eukaryotic cells?
A) lysosome
B) vacuole
C) mitochondrion
D) Golgi apparatus
E) peroxisome
C
Which organelle often takes up much of the volume of a plant cell?
A) lysosome
B) vacuole
C) mitochondrion
D) Golgi apparatus
E) peroxisome
B
The liver is involved in detoxification of many poisons and drugs. Which of the following structures is primarily involved in this process and therefore abundant in liver cells?
A) rough ER
B) smooth ER
C) Golgi apparatus
D) nuclear envelope
E) transport vesicles
B
Hydrolytic enzymes must be segregated and packaged to prevent general destruction of cellular components. Which of the following organelles contains these hydrolytic enzymes in animal cells?
A) chloroplast
B) lysosome
C) central vacuole
D) peroxisome
E) glyoxysome
B
Which structure is the site of the synthesis of proteins that may be exported from the cell?
A) rough ER
B) lysosomes
C) plasmodesmata
D) Golgi vesicles
E) free cytoplasmic ribosomes
A
Which type of organelle or structure is primarily involved in the synthesis of oils, phospholipids, and steroids?
A) ribosome
B) lysosome
C) smooth endoplasmic reticulum
D) mitochondrion
E) contractile vacuole
C
The nuclear lamina is an array of filaments on the inner side of the nuclear membrane. If a method were found that could cause the lamina to fall into disarray, what would you expect to be the most likely consequence?
A) the loss of all nuclear function
B) the inability of the nucleus to divide during cell division
C) a change in the shape of the nucleus
D) failure of chromosomes to carry genetic information
E) inability of the nucleus to keep out destructive chemicals
C
Some regions of the plasma membrane, called lipid rafts, have a higher concentration of cholesterol molecules. As a result, these lipid rafts
A) are more fluid than the surrounding membrane.
B) are more rigid than the surrounding membrane.
C) are able to flip from inside to outside.
D) detach from the plasma membrane and clog arteries.
E) have higher rates of lateral diffusion of lipids and proteins into and out of the lipid rafts.
B
Which of the following types of molecules are the major structural components of the cell membrane?
A) phospholipids and cellulose
B) nucleic acids and proteins
C) phospholipids and proteins
D) proteins and cellulose
E) glycoproteins and cholesterol
C
Which of the following is one of the ways that the membranes of winter wheat are able to remain fluid when it is extremely cold?
A) by increasing the percentage of unsaturated phospholipids in the membrane
B) by increasing the percentage of cholesterol molecules in the membrane
C) by decreasing the number of hydrophobic proteins in the membrane
D) by cotransport of glucose and hydrogen
E) by using active transport
A
In order for a protein to be an integral membrane protein it would have to be
A) hydrophilic.
B) hydrophobic.
C) amphipathic, with at least one hydrophobic region.
D) completely covered with phospholipids.
E) exposed on only one surface of the membrane.
C
Which of the following is a reasonable explanation for why unsaturated fatty acids help keep any membrane more fluid at lower temperatures?
A) The double bonds form kinks in the fatty acid tails, preventing adjacent lipids from packing tightly.
B) Unsaturated fatty acids have a higher cholesterol content and therefore more cholesterol in membranes.
C) Unsaturated fatty acids are more polar than saturated fatty acids.
D) The double bonds block interaction among the hydrophilic head groups of the lipids.
E) The double bonds result in shorter fatty acid tails and thinner membranes.
A
An animal cell lacking oligosaccharides on the external surface of its plasma membrane would likely be impaired in which function?
A) transporting ions against an electrochemical gradient
B) cell-cell recognition
C) maintaining fluidity of the phospholipid bilayer
D) attaching to the cytoskeleton
E) establishing the diffusion barrier to charged molecules
B
Which of these are not embedded in the hydrophobic portion of the lipid bilayer at all?
A) transmembrane proteins
B) integral proteins
C) peripheral proteins
D) integrins
E) glycoproteins
C
What kinds of molecules pass through a cell membrane most easily?
A) large and hydrophobic
B) small and hydrophobic
C) large polar
D) ionic
E) monosaccharides such as glucose
B
Nitrous oxide gas molecules diffusing across a cell's plasma membrane is an example of
A) diffusion across the lipid bilayer.
B) facilitated diffusion.
C) active transport.
D) osmosis.
E) cotransport.
A
Which of the following would likely move through the lipid bilayer of a plasma membrane most rapidly?
A) CO₂
B) an amino acid
C) glucose
D) K⁺
E) starch
A
Which of the following statements is correct about diffusion?
A) It is very rapid over long distances.
B) It requires an expenditure of energy by the cell.
C) It is a passive process in which molecules move from a region of higher concentration to a region of lower concentration.
D) It is an active process in which molecules move from a region of lower concentration to one of higher concentration.
E) It requires integral proteins in the cell membrane.
C
Water passes quickly through cell membranes because
A) the bilayer is hydrophilic.
B) it moves through hydrophobic channels.
C) water movement is tied to ATP hydrolysis.
D) it is a small, polar, charged molecule.
E) it moves through aquaporins in the membrane.
E
Mammalian blood contains the equivalent of 0.15 M NaCl. Seawater contains the equivalent of 0.45 M NaCl. What will happen if red blood cells are transferred to seawater?
A) Water will leave the cells, causing them to shrivel and collapse.
B) NaCl will be exported from the red blood cells by facilitated diffusion.
C) The blood cells will take up water, swell, and eventually burst.
D) NaCl will passively diffuse into the red blood cells.
E) The blood cells will expend ATP for active transport of NaCl into the cytoplasm.
A
Which of the following membrane activities require energy from ATP hydrolysis?
A) facilitated diffusion of chloride ions across the membrane through a chloride channel
B) movement of water into a cell
C) Na⁺ ions moving out of a mammalian cell bathed in physiological saline
D) movement of glucose molecules into a bacterial cell from a medium containing a higher concentration of glucose than inside the cell
E) movement of carbon dioxide out of a paramecium
C
Glucose diffuses slowly through artificial phospholipid bilayers. The cells lining the small intestine, however, rapidly move large quantities of glucose from the glucose-rich food into their glucose-poor cytoplasm. Using this information, which transport mechanism is most probably functioning in the intestinal cells?
A) simple diffusion
B) phagocytosis
C) active transport pumps
D) exocytosis
E) facilitated diffusion
E
Which of the following is most likely true of a protein that cotransports glucose and sodium ions into the intestinal cells of an animal?
A) The sodium ions are moving down their electrochemical gradient while glucose is moving up.
B) Glucose entering the cell along its concentration gradient provides energy for uptake of sodium ions against the electrochemical gradient.
C) Sodium ions can move down their electrochemical gradient through the cotransporter whether or not glucose is present outside the cell.
D) The cotransporter can also transport potassium ions.
E) A substance that blocks sodium ions from binding to the cotransport protein will also block the transport of glucose.
E
Ions diffuse across membranes through specific ion channels
A) down their chemical gradients.
B) down their concentration gradients.
C) down the electrical gradients.
D) down their electrochemical gradients.
E) down the osmotic potential gradients.
D
An organism with a cell wall would most likely be unable to take in materials through
A) diffusion.
B) osmosis.
C) active transport.
D) phagocytosis.
E) facilitated diffusion.
D
The difference between pinocytosis and receptor-mediated endocytosis is that
A) pinocytosis brings only water molecules into the cell, but receptor-mediated endocytosis brings in other molecules as well.
B) pinocytosis increases the surface area of the plasma membrane whereas receptor-mediated endocytosis decreases the plasma membrane surface area.
C) pinocytosis is nonselective in the molecules it brings into the cell, whereas receptor-mediated endocytosis offers more selectivity.
D) pinocytosis requires cellular energy, but receptor-mediated endocytosis does not.
E) pinocytosis can concentrate substances from the extracellular fluid, but receptor-mediated endocytosis cannot.
C
A bacterium engulfed by a white blood cell through phagocytosis will be digested by enzymes contained in
A) peroxisomes.
B) lysosomes.
C) Golgi vesicles.
D) vacuoles.
E) secretory vesicles.
B
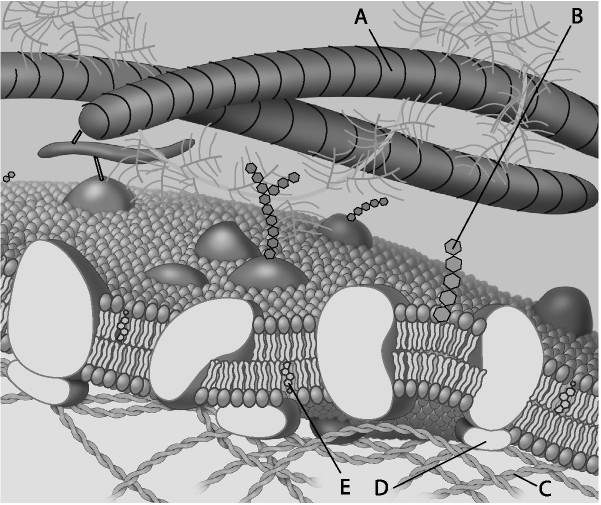
Which component is the peripheral protein?
A) A
B) B
C) C
D) D
E) E
D
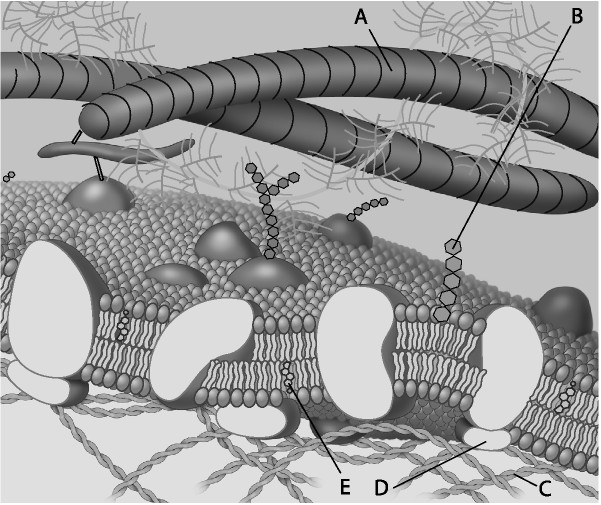
Which component is cholesterol?
A) A
B) B
C) C
D) D
E) E
E
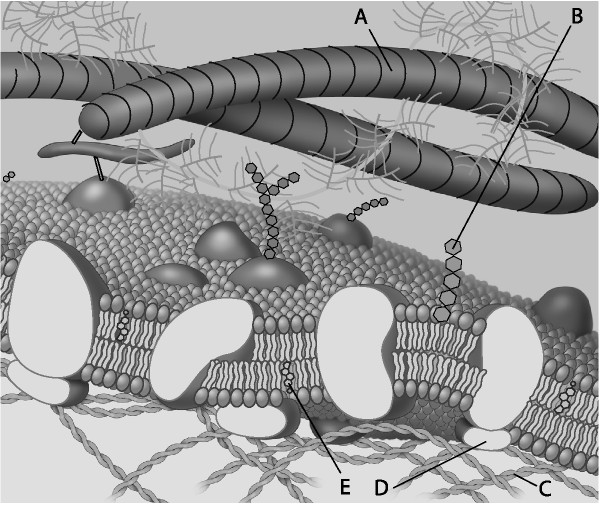
Which component is the fiber of the extracellular matrix?
A) A
B) B
C) C
D) D
E) E
A
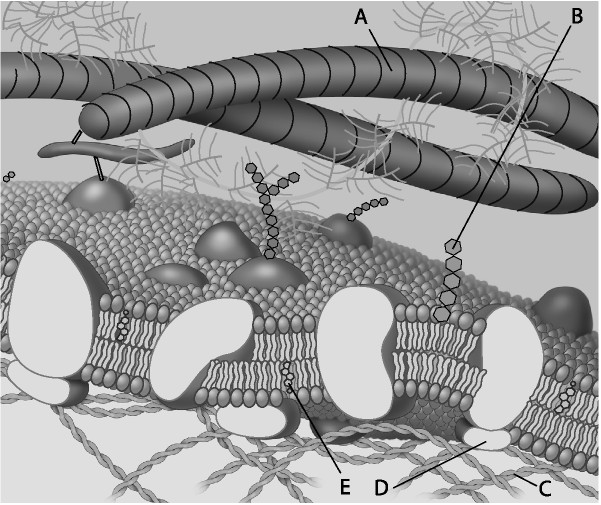
Which component is a microfilament of the cytoskeleton?
A) A
B) B
C) C
D) D
E) E
C
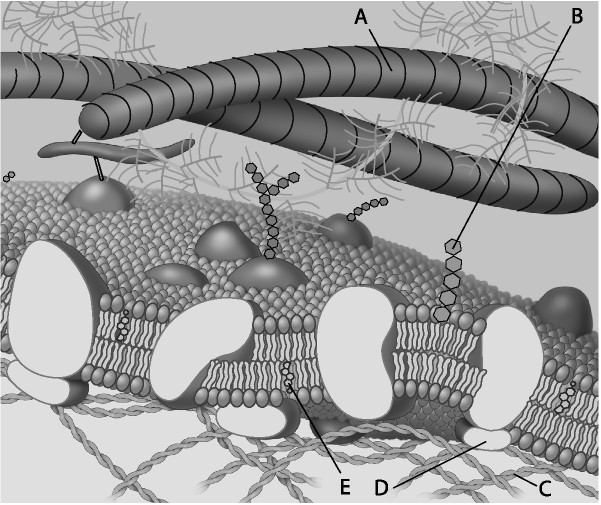
Which component is a glycolipid?
A) A
B) B
C) C
D) D
E) E
B
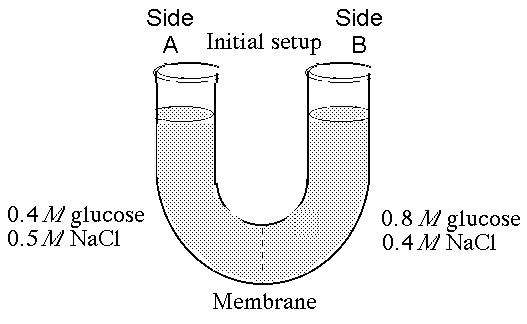
The solutions in the arms of a U-tube are separated at the bottom of the tube by a selectively permeable membrane. The membrane is permeable to sodium chloride but not to glucose. Side A is filled with a solution of 0.4 M glucose and 0.5 M sodium chloride (NaCl), and side B is filled with a solution containing 0.8 M glucose and 0.4 M sodium chloride. Initially, the volume in both arms is the same.
At the beginning of the experiment,
A) side A is hypertonic to side B.
B) side A is hypotonic to side B.
C) side A is isotonic to side B.
D) side A is hypertonic to side B with respect to glucose.
E) side A is hypotonic to side B with respect to sodium chloride.
B
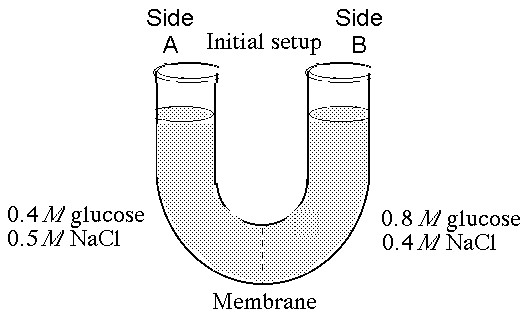
If you examine side A after three days, you should find
A) a decrease in the concentration of NaCl and glucose and an increase in the water level.
B) a decrease in the concentration of NaCl, an increase in water level, and no change in the concentration of glucose.
C) no net change in the system.
D) a decrease in the concentration of NaCl and a decrease in the water level.
E) no change in the concentration of NaCl and glucose and an increase in the water level.
D
You are working on a team that is designing a new drug. In order for this drug to work, it must enter the cytoplasm of specific target cells. Which of the following would be a factor that determines whether the molecule selectively enters the target cells?
A) blood or tissue type of the patient
B) hydrophobicity of the drug molecule
C) lack of charge on the drug molecule
D) similarity of the drug molecule to other molecules transported by the target cells
E) lipid composition of the target cells' plasma membrane
D
Whenever energy is transformed, there is always an increase in the
A) free energy of the system.
B) free energy of the universe.
C) entropy of the system.
D) entropy of the universe.
E) enthalpy of the universe.
D
Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the second law of thermodynamics?
A) Living organisms do not obey the second law of thermodynamics, which states that entropy must increase with time.
B) Life obeys the second law of thermodynamics because the decrease in entropy as the organism grows is exactly balanced by an increase in the entropy of the universe.
C) Living organisms do not follow the laws of thermodynamics.
D) As a consequence of growing, organisms cause a greater increase in entropy in their environment than the decrease in entropy associated with their growth.
E) Living organisms are able to transform energy into entropy.
D
Which of the following types of reactions would decrease the entropy within a cell?
A) anabolic reactions
B) hydrolysis
C) respiration
D) digestion
E) catabolic reactions
A
A chemical reaction that has a positive ΔG is correctly described as
A) endergonic.
B) endothermic.
C) enthalpic.
D) spontaneous.
E) exothermic.
A
Why is ATP an important molecule in metabolism?
A) Its hydrolysis provides an input of free energy for exergonic reactions.
B) It provides energy coupling between exergonic and endergonic reactions.
C) Its terminal phosphate group contains a strong covalent bond that, when hydrolyzed, releases free energy.
D) Its terminal phosphate bond has higher energy than the other two.
E) It is one of the four building blocks for DNA synthesis.
B
Which of the following statements is true concerning catabolic pathways?
A) They combine molecules into more energy-rich molecules.
B) They supply energy, primarily in the form of ATP, for the cell's work.
C) They are endergonic.
D) They are spontaneous and do not need enzyme catalysis.
E) They build up complex molecules such as protein from simpler compounds.
B
Which of the following statements regarding enzymes is true?
A) Enzymes increase the rate of a reaction by making the reaction more exergonic.
B) Enzymes increase the rate of a reaction by lowering the activation energy barrier.
C) Enzymes increase the rate of a reaction by reducing the rate of reverse reactions.
D) Enzymes change the equilibrium point of the reactions they catalyze.
E) Enzymes make the rate of a reaction independent of substrate concentrations.
B
The active site of an enzyme is the region that
A) binds allosteric regulators of the enzyme.
B) is involved in the catalytic reaction of the enzyme.
C) binds noncompetitive inhibitors of the enzyme.
D) is inhibited by the presence of a coenzyme or a cofactor.
B
Some of the drugs used to treat HIV patients are competitive inhibitors of the HIV reverse transcriptase enzyme. Unfortunately, the high mutation rate of HIV means that the virus rapidly acquires mutations with amino acid changes that make them resistant to these competitive inhibitors. Where in the reverse transcriptase enzyme would such amino acid changes most likely occur in drug-resistant viruses?
A) in or near the active site
B) at an allosteric site
C) at a cofactor binding site
D) in regions of the protein that determine packaging into the virus capsid
E) such mutations could occur anywhere with equal probability
A
Protein kinases are enzymes that transfer the terminal phosphate from ATP to an amino acid residue on the target protein. Many are located on the plasma membrane as integral membrane proteins or peripheral membrane proteins. What purpose may be served by their plasma membrane localization?
A) ATP is more abundant near the plasma membrane.
B) They can more readily encounter and phosphorylate other membrane proteins.
C) Membrane localization lowers the activation energy of the phosphorylation reaction.
D) They flip back and forth across the membrane to access target proteins on either side.
E) They require phospholipids as a cofactor.
B
When you have a severe fever, what grave consequence may occur if the fever is not controlled?
A) destruction of your enzymes' primary structure
B) removal of amine groups from your proteins
C) change in the tertiary structure of your enzymes
D) removal of the amino acids in active sites of your enzymes
E) binding of your enzymes to inappropriate substrates
C
How does a noncompetitive inhibitor decrease the rate of an enzyme reaction?
A) by binding at the active site of the enzyme
B) by changing the shape of the enzyme's active site
C) by changing the free energy change of the reaction
D) by acting as a coenzyme for the reaction
E) by decreasing the activation energy of the reaction
B
Protein kinases are enzymes that catalyze phosphorylation of target proteins at specific sites, whereas protein phosphatases catalyze removal of phosphate(s) from phosphorylated proteins. Phosphorylation and dephosphorylation can function as an on-off switch for a protein's activity, most likely through
A) the change in a protein's charge leading to a conformational change.
B) the change in a protein's charge leading to cleavage.
C) a change in the optimal pH at which a reaction will occur.
D) a change in the optimal temperature at which a reaction will occur.
E) the excision of one or more peptides.
A
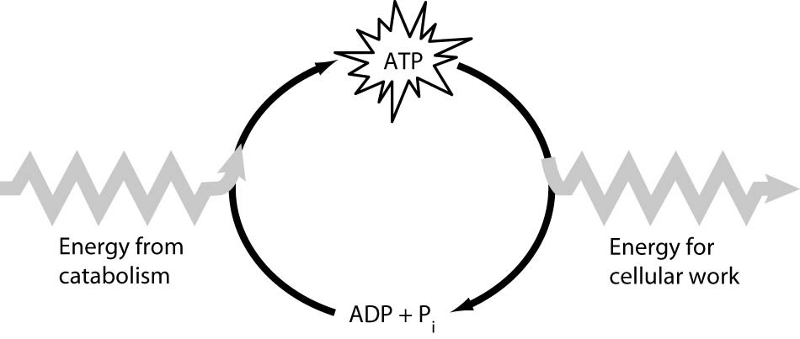
Which of the following is the most correct interpretation of the figure?
A) Inorganic phosphate is created from organic phosphate.
B) Energy from catabolism can be used directly for performing cellular work.
C) ADP + Pi are a set of molecules that store energy for catabolism.
D) ATP is a molecule that acts as an intermediary to store energy for cellular work.
E) Pi acts as a shuttle molecule to move energy from ATP to ADP.
D
Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
Based on this information, which of the following is correct?
A) Succinate dehydrogenase is the enzyme, and fumarate is the substrate.
B) Succinate dehydrogenase is the enzyme, and malonic acid is the substrate.
C) Succinate is the substrate, and fumarate is the product.
D) Fumarate is the product, and malonic acid is a noncompetitive inhibitor.
E) Malonic acid is the product, and fumarate is a competitive inhibitor.
C
Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
What is malonic acid's role with respect to succinate dehydrogenase?
A) It is a competitive inhibitor.
B) It blocks the binding of fumarate.
C) It is a noncompetitive inhibitor.
D) It is able to bind to succinate.
E) It is an allosteric regulator.
A
Which of the following is characterized by a cell releasing a signal molecule into the environment, followed by a number of cells in the immediate vicinity responding?
A) hormonal signaling
B) autocrine signaling
C) paracrine signaling
D) endocrine signaling
E) synaptic signaling
C
When a neuron responds to a particular neurotransmitter by opening gated ion channels, the neurotransmitter is serving as which part of the signal pathway?
A) receptor
B) relay molecule
C) transducer
D) signal molecule
E) endocrine molecule
D
Testosterone functions inside a cell by
A) acting as a signal receptor that activates tyrosine kinases.
B) binding with a receptor protein that enters the nucleus and activates specific genes.
C) acting as a steroid signal receptor that activates ion channel proteins.
D) becoming a second messenger that inhibits adenylyl cyclase.
E) coordinating a phosphorylation cascade that increases spermatogenesis.
B
One of the major categories of receptors in the plasma membrane reacts by forming dimers, adding phosphate groups, and then activating relay proteins. Which type does this?
A) G protein-coupled receptors
B) ligand-gated ion channels
C) steroid receptors
D) receptor tyrosine kinases
D
Since steroid receptors are located intracellularly, which of the following is true?
A) The receptor molecules are themselves lipids or glycolipids.
B) The steroid/receptor complex can cross the nuclear membrane.
C) The unbound steroid receptors are quickly recycled by lysosomes.
D) The concentration of steroid receptors must be relatively high in most cells.
E) The receptor molecules are free to move in and out of most organelles.
B
In general, a signal transmitted via phosphorylation of a series of proteins
A) brings a conformational change to each protein.
B) requires binding of a hormone to a cytosol receptor.
C) cannot occur in yeasts because they lack protein phosphatases.
D) requires phosphorylase activity.
E) allows target cells to change their shape and therefore their activity.
A
Which of the following would be inhibited by a drug that specifically blocks the addition of phosphate groups to proteins?
A) G protein-coupled receptor signaling
B) ligand-gated ion channel signaling
C) adenylyl cyclase activity
D) phosphatase activity
E) receptor tyrosine kinase activity
E
At puberty, an adolescent female body changes in both structure and function of several organ systems, primarily under the influence of changing concentrations of estrogens and other steroid hormones. How can one hormone, such as estrogen, mediate so many effects?
A) Estrogen is produced in very large concentration and therefore diffuses widely.
B) Estrogen has specific receptors inside several cell types, but each cell responds in the same way to its binding.
C) Estrogen is kept away from the surface of any cells not able to bind it at the surface.
D) Estrogen binds to specific receptors inside many kinds of cells, each of which have different responses to its binding.
E) The subcomponents of estrogen, when metabolized, can influence cell response.
D
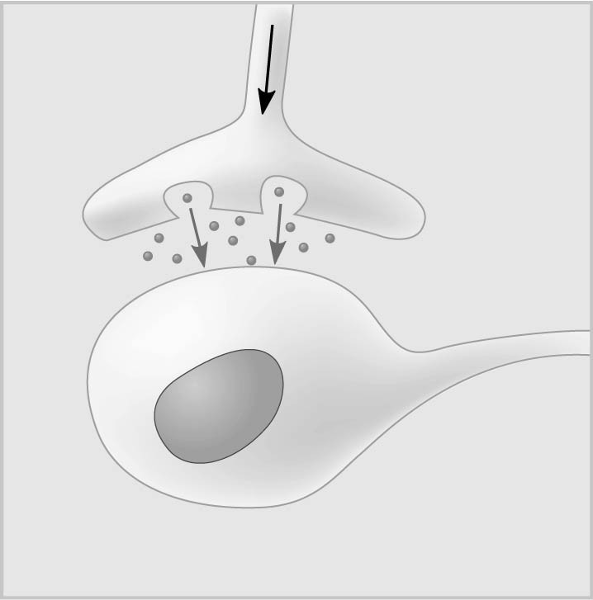
Which of the following types of signaling is represented in the figure?
A) autocrine
B) paracrine
C) hormonal
D) synaptic
E) long distance
D
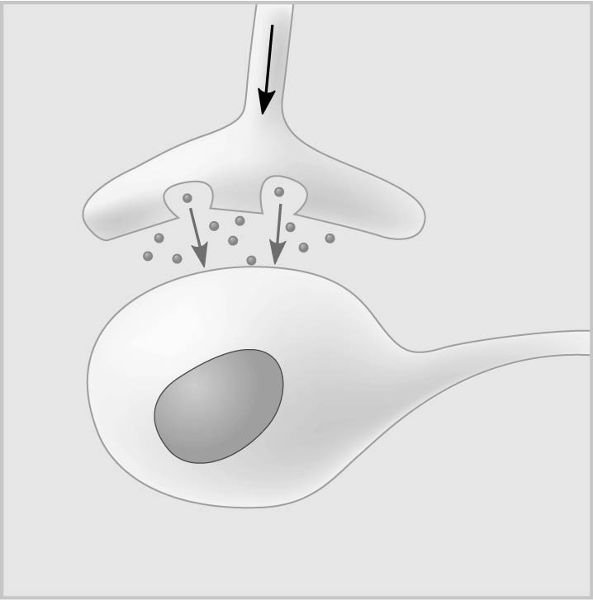
In the figure, the dots in the space between the two structures represent which of the following?
A) receptor molecules
B) signal transducers
C) neurotransmitters
D) hormones
E) pheromones
C
Humans have receptors for two kinds of beta adrenergic compounds such as catecholamines to control cardiac muscle contractions. Some are beta 1 receptors that promote increased heart rate. Other drugs, called beta blockers, slow heart rate. Smooth muscle cells, however, have beta 2 receptors, which mediate muscle relaxation. Blockers of these effects are sometimes used to treat asthma.
The description above illustrates which of the following?
A) Just because a drug acts on one type of receptor does not mean that it will act on another type.
B) Beta blockers can be used effectively on any type of muscle.
C) Beta adrenergic receptors must be in the cytosol if they are going to influence contraction and relaxation.
D) The chemical structures of the beta 1 and beta 2 receptors must have the same active sites.
A
Humans have receptors for two kinds of beta adrenergic compounds such as catecholamines to control cardiac muscle contractions. Some are beta 1 receptors that promote increased heart rate. Other drugs, called beta blockers, slow heart rate. Smooth muscle cells, however, have beta 2 receptors, which mediate muscle relaxation. Blockers of these effects are sometimes used to treat asthma.
The use of beta 2 antagonist drugs may be useful in asthma because they may
A) increase constriction of the skeletal muscle of the chest wall.
B) increase heart rate and therefore allow the patient to get more oxygen circulated.
C) dilate the bronchioles by relaxing their smooth muscle.
D) override the beta blockers that the patient is already taking.
E) obstruct all G protein-mediated receptors.
C
Humans have receptors for two kinds of beta adrenergic compounds such as catecholamines to control cardiac muscle contractions. Some are beta 1 receptors that promote increased heart rate. Other drugs, called beta blockers, slow heart rate. Smooth muscle cells, however, have beta 2 receptors, which mediate muscle relaxation. Blockers of these effects are sometimes used to treat asthma.
Beta 2 antagonist drugs might also be used most effectively for which of the following?
A) cardiac arrhythmias
B) increased gastric acid production
C) neuropathy of the extremities
D) increasing low blood pressure
E) decreasing peristalsis
D
What is the term for metabolic pathways that release stored energy by breaking down complex molecules?
A) anabolic pathways
B) catabolic pathways
C) fermentation pathways
D) thermodynamic pathways
E) bioenergetic pathways
B
Why does the oxidation of organic compounds by molecular oxygen to produce CO₂ and water release free energy?
A) The covalent bonds in organic molecules and molecular oxygen have more kinetic energy than the covalent bonds in water and carbon dioxide.
B) Electrons are being moved from atoms that have a lower affinity for electrons (such as C) to atoms with a higher affinity for electrons (such as O).
C) The oxidation of organic compounds can be used to make ATP.
D) The electrons have a higher potential energy when associated with water and CO₂ than they do in organic compounds.
E) The covalent bond in O₂ is unstable and easily broken by electrons from organic molecules.
B
When a molecule of NAD⁺ (nicotinamide adenine dinucleotide) gains a hydrogen atom (not a proton), the molecule becomes
A) dehydrogenated.
B) oxidized.
C) reduced.
D) redoxed.
E) hydrolyzed.
C
Which of the following statements describes NAD⁺?
A) NAD⁺ is reduced to NADH during glycolysis, pyruvate oxidation, and the citric acid cycle.
B) NAD⁺ has more chemical energy than NADH.
C) NAD⁺ is oxidized by the action of hydrogenases.
D) NAD⁺ can donate electrons for use in oxidative phosphorylation.
E) In the absence of NAD⁺, glycolysis can still function.
A
Where does glycolysis take place in eukaryotic cells?
A) mitochondrial matrix
B) mitochondrial outer membrane
C) mitochondrial inner membrane
D) mitochondrial intermembrane space
E) cytosol
E
The oxygen consumed during cellular respiration is involved directly in which process or event?
A) glycolysis
B) accepting electrons at the end of the electron transport chain
C) the citric acid cycle
D) the oxidation of pyruvate to acetyl CoA
E) the phosphorylation of ADP to form ATP
B
In addition to ATP, what are the end products of glycolysis?
A) CO₂ and H₂O
B) CO₂ and pyruvate
C) NADH and pyruvate
D) CO₂ and NADH
E) H₂O, FADH₂, and citrate
C
Starting with one molecule of glucose, the energy-containing products of glycolysis are
A) 2 NAD⁺, 2 pyruvate, and 2 ATP.
B) 2 NADH, 2 pyruvate, and 2 ATP.
C) 2 FADH₂, 2 pyruvate, and 4 ATP.
D) 6 CO₂, 2 ATP, and 2 pyruvate.
E) 6 CO₂, 30 ATP, and 2 pyruvate.
B
In glycolysis, for each molecule of glucose oxidized to pyruvate
A) two molecules of ATP are used and two molecules of ATP are produced.
B) two molecules of ATP are used and four molecules of ATP are produced.
C) four molecules of ATP are used and two molecules of ATP are produced.
D) two molecules of ATP are used and six molecules of ATP are produced.
E) six molecules of ATP are used and six molecules of ATP are produced.
B
A molecule that is phosphorylated
A) has been reduced as a result of a redox reaction involving the loss of an inorganic phosphate.
B) has a decreased chemical reactivity; it is less likely to provide energy for cellular work.
C) has been oxidized as a result of a redox reaction involving the gain of an inorganic phosphate.
D) has an increased chemical potential energy; it is primed to do cellular work.
E) has less energy than before its phosphorylation and therefore less energy for cellular work.
D
Why is glycolysis described as having an investment phase and a payoff phase?
A) It both splits molecules and assembles molecules.
B) It attaches and detaches phosphate groups.
C) It uses glucose and generates pyruvate.
D) It shifts molecules from cytosol to mitochondrion.
E) It uses stored ATP and then forms a net increase in ATP.
E
During cellular respiration, acetyl CoA accumulates in which location?
A) cytosol
B) mitochondrial outer membrane
C) mitochondrial inner membrane
D) mitochondrial intermembrane space
E) mitochondrial matrix
E
A young animal has never had much energy. He is brought to a veterinarian for help and is sent to the animal hospital for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactate than normal. Of the following, which is the best explanation of his condition?
A) His mitochondria lack the transport protein that moves pyruvate across the outer mitochondrial membrane.
B) His cells cannot move NADH from glycolysis into the mitochondria.
C) His cells contain something that inhibits oxygen use in his mitochondria.
D) His cells lack the enzyme in glycolysis that forms pyruvate.
E) His cells have a defective electron transport chain, so glucose goes to lactate instead of to acetyl CoA.
A
Where are the proteins of the electron transport chain located?
A) cytosol
B) mitochondrial outer membrane
C) mitochondrial inner membrane
D) mitochondrial intermembrane space
E) mitochondrial matrix
C
The primary role of oxygen in cellular respiration is to
A) yield energy in the form of ATP as it is passed down the respiratory chain.
B) act as an acceptor for electrons and hydrogen, forming water.
C) combine with carbon, forming CO₂.
D) combine with lactate, forming pyruvate.
E) catalyze the reactions of glycolysis.
B
Inside an active mitochondrion, most electrons follow which pathway?
A) glycolysis → NADH → oxidative phosphorylation → ATP → oxygen
B) citric acid cycle → FADH₂ → electron transport chain → ATP
C) electron transport chain → citric acid cycle → ATP → oxygen
D) pyruvate → citric acid cycle → ATP → NADH → oxygen
E) citric acid cycle → NADH → electron transport chain → oxygen
E
During aerobic respiration, H₂O is formed. Where does the oxygen atom for the formation of the water come from?
A) carbon dioxide (CO₂)
B) glucose (C₆H₁₂O₆)
C) molecular oxygen (O₂)
D) pyruvate (C₃H₃O₃-)
E) lactate (C₃H₅O₃-)
C
Energy released by the electron transport chain is used to pump H⁺ into which location in eukaryotic cells?
A) cytosol
B) mitochondrial outer membrane
C) mitochondrial inner membrane
D) mitochondrial intermembrane space
E) mitochondrial matrix
D
Where is ATP synthase located in the mitochondrion?
A) cytosol
B) electron transport chain
C) outer membrane
D) inner membrane
E) mitochondrial matrix
D
What is proton-motive force?
A) the force required to remove an electron from hydrogen
B) the force exerted on a proton by a transmembrane proton concentration gradient
C) the force that moves hydrogen into the intermembrane space
D) the force that moves hydrogen into the mitochondrion
E) the force that moves hydrogen to NAD⁺
B
In liver cells, the inner mitochondrial membranes are about five times the area of the outer mitochondrial membranes. What purpose must this serve?
A) It allows for an increased rate of glycolysis.
B) It allows for an increased rate of the citric acid cycle.
C) It increases the surface for oxidative phosphorylation.
D) It increases the surface for substrate-level phosphorylation.
E) It allows the liver cell to have fewer mitochondria.
C
In the absence of oxygen, yeast cells can obtain energy by fermentation, resulting in the production of
A) ATP, CO₂, and ethanol (ethyl alcohol).
B) ATP, CO₂, and lactate.
C) ATP, NADH, and pyruvate.
D) ATP, pyruvate, and oxygen.
E) ATP, pyruvate, and acetyl CoA.
A
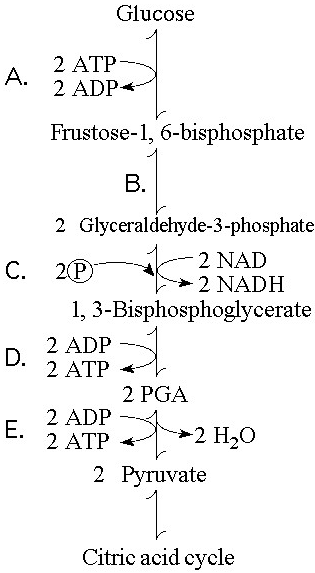
Which step in Figure 9.1 shows a split of one molecule into two smaller molecules?
A) A
B) B
C) C
D) D
E) E
B
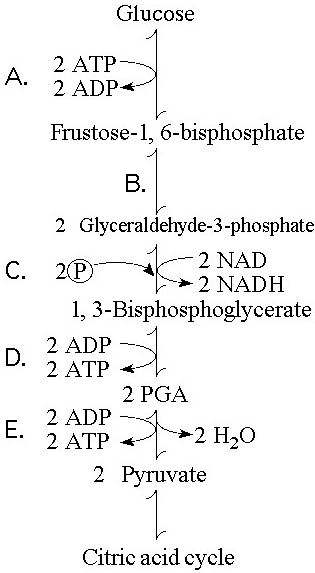
In which step in Figure 9.1 is an inorganic phosphate added to the reactant?
A) A
B) B
C) C
D) D
E) E
C
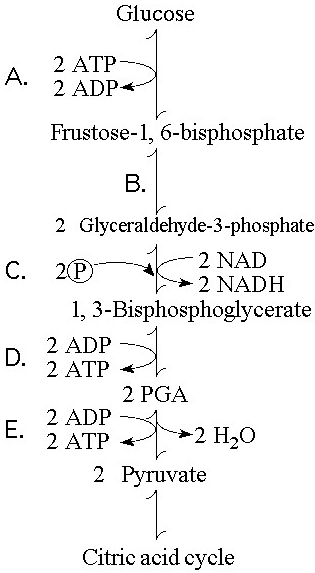
Which step in Figure 9.1 is a redox reaction?
A) A
B) B
C) C
D) D
E) E
C
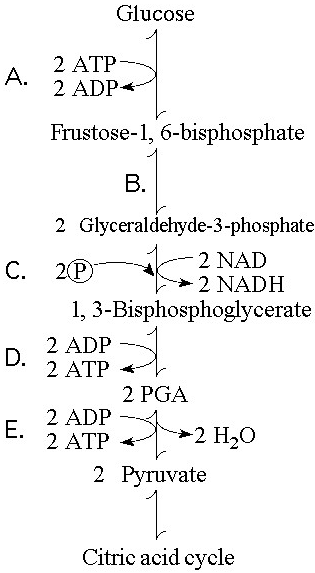
Which portion of the pathway in Figure 9.1 involves an endergonic reaction?
A) A
B) B
C) C
D) D
E) E
A
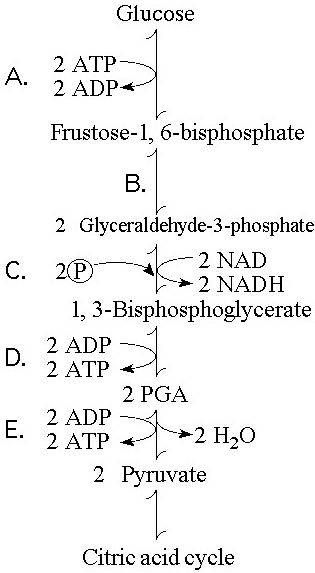
Which portion of the pathway in Figure 9.1 contains a phosphorylation reaction in which ATP is the phosphate source?
A) A
B) B
C) C
D) D
E) E
A
Where does the Calvin cycle take place?
A) stroma of the chloroplast
B) thylakoid membrane
C) cytoplasm surrounding the chloroplast
D) interior of the thylakoid (thylakoid space)
E) outer membrane of the chloroplast
A
When oxygen is released as a result of photosynthesis, it is a direct by-product of
A) reducing NADP⁺.
B) splitting water molecules.
C) chemiosmosis.
D) the electron transfer system of photosystem I.
E) the electron transfer system of photosystem II.
B
In the thylakoid membranes, what is the main role of the antenna pigment molecules?
A) split water and release oxygen to the reaction-center chlorophyll
B) harvest photons and transfer light energy to the reaction-center chlorophyll
C) synthesize ATP from ADP and Pi
D) transfer electrons to ferredoxin and then NADPH
E) concentrate photons within the stroma
B
Which of the events listed below occurs in the light reactions of photosynthesis?
A) NADP is produced.
B) NADPH is reduced to NADP⁺.
C) Carbon dioxide is incorporated into PGA.
D) ATP is phosphorylated to yield ADP.
E) Light is absorbed and funneled to reaction-center chlorophyll a.
E
Which statement describes the functioning of photosystem II?
A) Light energy excites electrons in the thylakoid membrane electron transport chain.
B) Photons are passed along to a reaction-center chlorophyll.
C) The P680 chlorophyll donates a pair of protons to NADP⁺, which is thus converted to NADPH.
D) The electron vacancies in P680⁺ are filled by electrons derived from water.
E) The splitting of water yields molecular carbon dioxide as a by-product.
D
Which of the following are directly associated with photosystem I?
A) harvesting of light energy by ATP
B) receiving electrons from the thylakoid membrane electron transport chain
C) generation of molecular oxygen
D) extraction of hydrogen electrons from the splitting of water
E) passing electrons to the thylakoid membrane electron transport chain
B
Some photosynthetic organisms contain chloroplasts that lack photosystem II, yet are able to survive. The best way to detect the lack of photosystem II in these organisms would be
A) to determine if they have thylakoids in the chloroplasts.
B) to test for liberation of O₂ in the light.
C) to test for CO₂ fixation in the dark.
D) to do experiments to generate an action spectrum.
E) to test for production of either sucrose or starch.
B
What are the products of linear photophosphorylation?
A) heat and fluorescence
B) ATP and P700
C) ATP and NADPH
D) ADP and NADP
E) P700 and P680
C
What does the chemiosmotic process in chloroplasts involve?
A) establishment of a proton gradient across the thylakoid membrane
B) diffusion of electrons through the thylakoid membrane
C) reduction of water to produce ATP energy
D) movement of water by osmosis into the thylakoid space from the stroma
E) formation of glucose, using carbon dioxide, NADPH, and ATP
A
In a plant cell, where are the ATP synthase complexes located?
A) thylakoid membrane only
B) plasma membrane only
C) inner mitochondrial membrane only
D) thylakoid membrane and inner mitochondrial membrane
E) thylakoid membrane and plasma membrane
D
Which of the following statements best describes the relationship between photosynthesis and respiration?
A) Respiration runs the biochemical pathways of photosynthesis in reverse.
B) Photosynthesis stores energy in complex organic molecules, whereas respiration releases it.
C) Photosynthesis occurs only in plants and respiration occurs only in animals.
D) ATP molecules are produced in photosynthesis and used up in respiration.
E) Respiration is anabolic and photosynthesis is catabolic.
B
Reduction of NADP⁺ occurs during
A) photosynthesis.
B) respiration.
C) both photosynthesis and respiration.
D) neither photosynthesis nor respiration.
E) photorespiration.
A
Generation of proton gradients across membranes occurs during
A) photosynthesis.
B) respiration.
C) both photosynthesis and respiration.
D) neither photosynthesis nor respiration.
E) photorespiration.
C
What is the relationship between wavelength of light and the quantity of energy per photon?
A) They have a direct, linear relationship.
B) They are inversely related.
C) They are logarithmically related.
D) They are separate phenomena.
E) They are only related in certain parts of the spectrum.
B
Carotenoids are often found in foods that are considered to have antioxidant properties in human nutrition. What related function do they have in plants?
A) They serve as accessory pigments to increase light absorption.
B) They protect against oxidative damage from excessive light energy.
C) They shield the sensitive chromosomes of the plant from harmful ultraviolet radiation.
D) They reflect orange light and enhance red light absorption by chlorophyll.
E) They take up and remove toxins from the groundwater.
B
In a cyanobacterium, the reactions that produce NADPH occur in
A) the light reactions alone.
B) the Calvin cycle alone.
C) both the light reactions and the Calvin cycle.
D) neither the light reactions nor the Calvin cycle.
E) the chloroplast, but is not part of photosynthesis.
A
The reactions that produce molecular oxygen (O₂) take place in
A) the light reactions alone.
B) the Calvin cycle alone.
C) both the light reactions and the Calvin cycle.
D) neither the light reactions nor the Calvin cycle.
E) the chloroplast, but are not part of photosynthesis.
A
What is the primary function of the Calvin cycle?
A) use ATP to release carbon dioxide
B) use NADPH to release carbon dioxide
C) split water and release oxygen
D) transport RuBP out of the chloroplast
E) synthesize simple sugars from carbon dioxide
E
Reactions that require CO₂ take place in
A) the light reactions alone.
B) the Calvin cycle alone.
C) both the light reactions and the Calvin cycle.
D) neither the light reactions nor the Calvin cycle.
E) the chloroplast, but is not part of photosynthesis.
B
Photorespiration occurs when rubisco reacts RuBP with
A) CO₂.
B) O₂.
C) glyceraldehyde 3-phosphate.
D) 3-phosphoglycerate.
E) NADPH.
B
Why are C₄ plants able to photosynthesize with no apparent photorespiration?
A) They do not participate in the Calvin cycle.
B) They use PEP carboxylase to initially fix CO₂.
C) They are adapted to cold, wet climates.
D) They conserve water more efficiently.
E) They exclude oxygen from their tissues.
B
CAM plants keep stomata closed in daytime, thus reducing loss of water. They can do this because they
A) fix CO₂ into organic acids during the night.
B) fix CO₂ into sugars in the bundle-sheath cells.
C) fix CO₂ into pyruvate in the mesophyll cells.
D) use the enzyme phosphofructokinase, which outcompetes rubisco for CO₂.
E) use photosystem I and photosystem II at night
A
Figure 10.1 shows the absorption spectrum for chlorophyll a and the action spectrum for photosynthesis. Why are they different?
A) Green and yellow wavelengths inhibit the absorption of red and blue wavelengths.
B) Bright sunlight destroys photosynthetic pigments.
C) Oxygen given off during photosynthesis interferes with the absorption of light.
D) Other pigments absorb light in addition to chlorophyll a.
E) Aerobic bacteria take up oxygen, which changes the measurement of the rate of photosynthesis.
D
What wavelength of light in the figure is most effective in driving photosynthesis?
A) 420 mm
B) 475 mm
C) 575 mm
D) 625 mm
E) 730 mm
A
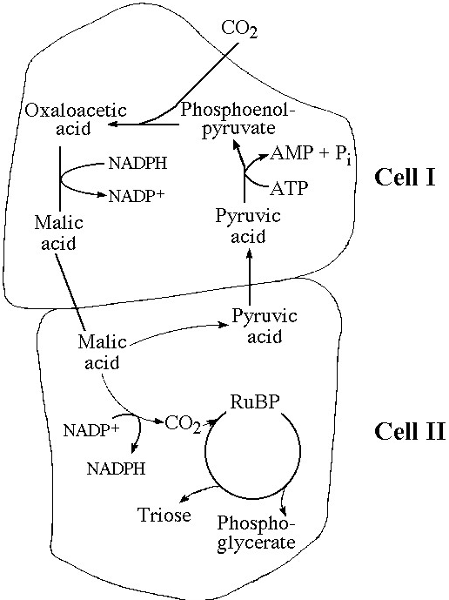
Which of the following statements is true concerning Figure 10.3?
A) It represents cell processes involved in C₄ photosynthesis.
B) It represents the type of cell structures found in CAM plants.
C) It represents an adaptation that maximizes photorespiration.
D) It represents a C₃ photosynthetic system.
E) It represents a relationship between plant cells that photosynthesize and those that cannot.
A
The centromere is a region in which
A) chromatids remain attached to one another until anaphase.
B) metaphase chromosomes become aligned at the metaphase plate.
C) chromosomes are grouped during telophase.
D) the nucleus is located prior to mitosis.
E) new spindle microtubules form at either end.
A
If there are 20 chromatids in a cell, how many centromeres are there?
A) 10
B) 20
C) 30
D) 40
E) 80
A
If cells in the process of dividing are subjected to colchicine, a drug that interferes with the formation of the spindle apparatus, at which stage will mitosis be arrested?
A) anaphase
B) prophase
C) telophase
D) metaphase
E) interphase
D
Where do the microtubules of the spindle originate during mitosis in both plant and animal cells?
A) centromere
B) centrosome
C) centriole
D) chromatid
E) kinetochore
B
Why do chromosomes coil during mitosis?
A) to increase their potential energy
B) to allow the chromosomes to move without becoming entangled and breaking
C) to allow the chromosomes to fit within the nuclear envelope
D) to allow the sister chromatids to remain attached
E) to provide for the structure of the centromere
B
During which phase of mitosis do the chromatids become chromosomes?
A) telophase
B) anaphase
C) prophase
D) metaphase
E) cytokinesis
A
What is a cleavage furrow?
A) a ring of vesicles forming a cell plate
B) the separation of divided prokaryotes
C) a groove in the plasma membrane between daughter nuclei
D) the metaphase plate where chromosomes attach to the spindle
E) the space that is created between two chromatids during anaphase
C
Why do neurons and some other specialized cells divide infrequently?
A) They no longer have active nuclei.
B) They no longer carry receptors for signal molecules.
C) They have been shunted into G₀.
D) They can no longer bind Cdk to cyclin.
E) They show a drop in MPF concentration.
C
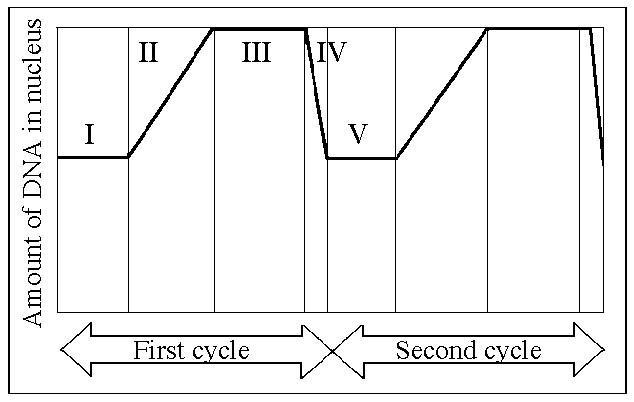
In the figure above, mitosis is represented by which numbered part(s) of the cycle?
A) I
B) II
C) III
D) IV
E) V
D
G₁ is represented by which numbered part(s) of the cycle?
A) I or V
B) II or IV
C) III only
D) IV only
E) V only
A
Which number represents DNA synthesis?
A) I
B) II
C) III
D) IV
E) V
B
Which number represents the point in the cell cycle during which the chromosomes are replicated?
A) I
B) II
C) III
D) IV
E) V
B