1. Elemental Mercury was first discovered when a mercury oxide was decomposed with heat, forming mercury metal and oxygen gas. When a 0.204-g sample of mercury oxide is heated, 0.189 of mercury metal remains. NOTE: Do not attempt this experiment in the laboratory because of the release of toxic mercury vapor.
a. What is the mole ration of mercury to oxygen in the sample?
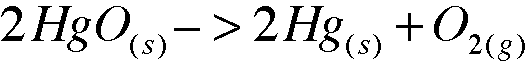
2:1
1. Elemental Mercury was first discovered when a mercury oxide was decomposed with heat, forming mercury metal and oxygen gas. When a 0.204-g sample of mercury oxide is heated, 0.189 of mercury metal remains. NOTE: Do not attempt this experiment in the laboratory because of the release of toxic mercury vapor.
b. What is the empirical formula of the mercury oxide?
HgO
2. A 5.90-g sample of titanium chemically combines with oxygen gas to form 9.84g go titanium oxide.
a. What is the empirical formula of titanium oxide?
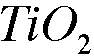
Titanium dioxide or titanium(IV) oxide
2. A 5.90-g sample of titanium chemically combines with oxygen gas to form 9.84g go titanium oxide.
b. What is the percent by mass of titanium and the percent by mass of oxygen in the sample?
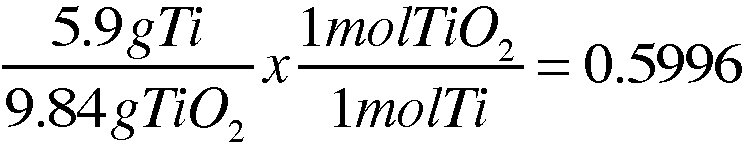
59.96% Ti
40.04% O_2
3. a. Experimental Procedure, Part A. List two reasons for using crucible tongs to handle the crucible tongs to handle the crucible and lid after their initial firing.
1: Crucible tongs are specifically designed to hold the crucible. Using the crucible tongs will minimize the risk of the crucible falling and burning the handler.
2: The crucible is extremely hot, so it is inadvisable to handle it with bare hands, latex gloves or clumsy mittens,
3. b. Why is it best to cool the crucible lid (and sample) in a desiccator rather than on the laboratory bench?
So that the laboratory bench is neither burned or damaged.
4. Experiment Procedure, Part D.2. State the reason for the use of the fume hood.
To avoid the inhalation of toxic, gaseous vapors caused by the reaction of nitric acid and tin.
5. Experiment Procedure, Part D.3. Characterize a cool flame.
A flame having maximal temperature below about 400 °C. If you can feel the heat of the flame with a hand held beside the crucible, the flame is too hot. The flame is usually entirely pale blue.
6. A sample of pure iron is covered with an excess of powdered elemental sulfur. The following data were collected:
- Mass of crucible and lid (g): 19.746
- Mass of iron, crucible, and lid (g): 20.422
The mixture was heated to a temperature where a reaction occurred and the excess sulfur was volatilized. Upon cooling, the following was recorded.
- Mass of compound, crucible, and lid (g): 21.195
Complete the following data analysis.
Mass of compound (g): 1.449g
21.195g - 19.746g = 1.446g
(Mass of compound, crucible, & lid AFTER reaction) - (Mass of crucible & lid) = (Mass of compound after reaction)
6. A sample of pure iron is covered with an excess of powdered elemental sulfur. The following data were collected:
- Mass of crucible and lid (g): 19.746
- Mass of iron, crucible, and lid (g): 20.422
The mixture was heated to a temperature where a reaction occurred and the excess sulfur was volatilized. Upon cooling, the following was recorded.
- Mass of compound, crucible, and lid (g): 21.195
Complete the following data analysis.
Mass of iron in the compound (g):
Fe + S = FeS
(Mass of compound, crucible, & lid) - (Mass of crucible & lid) = (Mass of iron)
20.422g - 19.746g = 0.676g
0.676g Fe
6. A sample of pure iron is covered with an excess of powdered elemental sulfur. The following data were collected:
- Mass of crucible and lid (g): 19.746
- Mass of iron, crucible, and lid (g): 20.422
The mixture was heated to a temperature where a reaction occurred and the excess sulfur was volatilized. Upon cooling, the following was recorded.
- Mass of compound, crucible, and lid (g): 21.195
Complete the following data analysis.
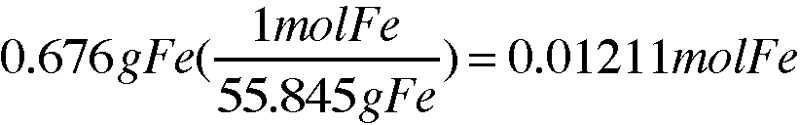
Moles of iron in compound (mol):
6. A sample of pure iron is covered with an excess of powdered elemental sulfur. The following data were collected:
- Mass of crucible and lid (g): 19.746
- Mass of iron, crucible, and lid (g): 20.422
The mixture was heated to a temperature where a reaction occurred and the excess sulfur was volatilized. Upon cooling, the following was recorded.
- Mass of compound, crucible, and lid (g): 21.195
Complete the following data analysis.
Mass of sulfur in the compound (g):
(mass of product) - (mass of iron) = (mass of sulfur)
1.449g - 0.676g = 0.773g
Feel free to drop a comment if you see anything wrong and I'll fix it as soon as possible. Don't forget to rate my helpfulness!
This book can be very vague, please, again, correct me where I've gone wrong.
6. A sample of pure iron is covered with an excess of powdered elemental sulfur. The following data were collected:
- Mass of crucible and lid (g): 19.746
- Mass of iron, crucible, and lid (g): 20.422
The mixture was heated to a temperature where a reaction occurred and the excess sulfur was volatilized. Upon cooling, the following was recorded.
- Mass of compound, crucible, and lid (g): 21.195
Complete the following data analysis.
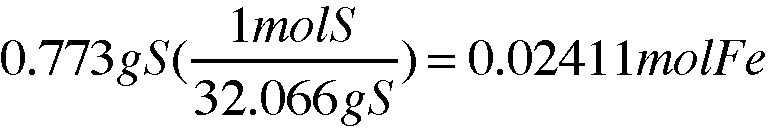
Moles of sulfur in the compound (mol):
6. A sample of pure iron is covered with an excess of powdered elemental sulfur. The following data were collected:
- Mass of crucible and lid (g): 19.746
- Mass of iron, crucible, and lid (g): 20.422
The mixture was heated to a temperature where a reaction occurred and the excess sulfur was volatilized. Upon cooling, the following was recorded.
- Mass of compound, crucible, and lid (g): 21.195
Complete the following data analysis.
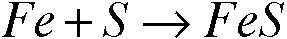
Empirical formula of the iron and sulfur compound: