2-1
An element is to a (an)_____as an organ is to a (an) _______
a atom; organism
b compound; organism
c molecule; cell
d atom; cell
e compound; organelle
2-2
In the term trace element, the modifier trace means
a the element is required in very small amounts
b the element can be used as a label to trace atoms through an organism's metabolism
c the element is very rare on earth
d the element enhances health but is not essential for the organism's long-term survival
e the element passes rapidly through the organism
2-3
Compared to 31P, the radioactive isotope 32 P has
a a different atomic number
b one more neutron
c one more proton
d one more electron
e a different charge
2-4
Atoms can be represented by simply listing the number of protons, neutrons, and electrons-for example, 2p+; 2n0; 2e- for helium. Which atom represents the 18O isotope of oxygen?
a 6p+; 8n0; 6e-
b 8p+; 10n0; 8e-
c 9p+; 9n0; 9e-
d 7p+; 2n0; 9e-
e 10p+; 8n0; 9e-
2-5
The atomic number of sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the electron configuration of sulfur, we can predict that the molecular formula of the compound will be
a HS
b HS2
c H2S
d H3S2
e H4S
2-6
Review the valences of carbon, oxygen, hydrogen, and nitrogen, and then determine which of the following molecules is most likely to exist.
a
O = C-H
b
H H
| |
H - O - C - C = O
|
H
c
H H
| |
H- C- H - C = O
|
H
d
O
|
H - N = H
2-7
The reactivity of an atom arises from
a the average distance of the outermost electron shell from the nucleus
b the existence of unpaired electrons in the valence shell
c the sum of the potential energies of all the elecron shells
d the potential energy of the valence shell
e the energy difference between the s and p orbitals
2-8 which of these statements is true of all anionic atoms
a the atom has more electrons that protons
b the atom has more protons than electrons
c the atom has fewer protons than does a neutral atom of the same element
d the atom has more neutrons than protons
e the net charge is 1-
2-9
What coefficients must be placed in the blanks so that all atoms are accounted for in the products?
C6H12O6 ____C2H6O + ____CO2
a 1;2
b 2;2
c 1;3
d 1;1
e 3;1
2-10
Which of the following statements correctly describes any chemical reaction that has reached equilibrium
a the concentration of products equals the concentration of reactants
b the rate of the forward reaction equals the rate of the reverse reaction
c both forward and reverse reactions have halted
d the reaction is now irreversible
e no reactants remain
3-1
what is the best explanation of the phrase "fitness of the environment," as used in this next chapter
a earth's environment is constant
b it is the physical environment, not life that has changed
c the environment of earth has adapted to life
d life as we know i depends on certain environmental qualities on earth
e water and other aspects of earths environment exist because they make the planet more suitable for life
3-2
Many mammals control their body temperature by sweating. Which property of water is most directly responsible for the ability of sweat to lower body temperature?
a water's change in density when it condenses
b water's ability to dissolve molecules in the air
c the release of heat by the formation of hydrogen bonds
d the absorption of heat by breaking of hydrogen bonds
e water's high surface tension
3-4
A slice of pizza has 500 kcal. If we could burn the pizza and use all the heat to warm a 50-L container of cold water, what would be the approximate increase in the temperature of the water? (note a liter of cold water weighs about 1 kg)
a 50 c
b 5 c
c 10 c
d 100 c
e 1 c
3-5
The bonds that are broken when water vaporizes are
a ionic bonds
b bonds between water molecules
c bonds between atoms within individual water molecules
d polar covalent bonds
e nonpolar covalent bonds
3-6
which of the following is an example of a hydrophobic material?
a paper
b table salt
c wax
d sugar
e pasta
3-7
We can be sure that a mole of table sugar and a mole of vitamin C are equal in their
a mass in daltons
b mass in grams
c number of moleculeser of atoms
d number of atoms
e volume
3-8
How many grams of acetic acid (C2H4O2) would you use to make 10 L of a .1 M aqueous solution of acetic acid? (note the atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 18 for oxygen.)
a 10g
b .1g
c 6g
d 60g
.6g
3-9
Acid precipitation has lowered the pH of a particular lake to 4.0. What is the hydrogen ion concentration of the lake?
a 4.0M
b 10^-10M
c 10^-4M
d 10^4M
e 10M
3-10
What is the hydroxide ion concentration of the lake described in question 9?
a 10^-7M
b 10^-4M
c 10^-10M
d 10^-14M
e 10M
4-1
organic chemistry is currently defined as
a the study of compounds that can be made only by living cells
b the study of carbon compounds
c the study of vital forces
d the study of natural (as opposed to synthetic)compounds
e the study of hydrocarbons
4-2
Choose the pair of terms that correctly completes this sentence: hydroxyl is to _____ as ____ is to aldehyde.
a carbonyl;ketone
b oxygen;carbon
c alcohol;carbonyl
d amine;carboxyl
e alcohol;ketone
4-3
Which of the following hydrocarbons has a double bond in its carbon skeleton?
a C3H8
b C2H6
c CH4
d C2H4
e C2H2
4-4
the gasoline consumed by an automobile is a fossil fuel consisting mostly of
a aldehydes
b amino acids
c alcohols
d hydrocarbons
e thiols
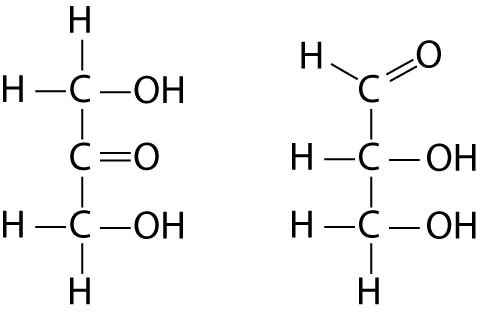
4-5
Choose the term that correctly describes the relationship between these 2 sugar molecules:
a structural isomers
b geometric isomers
c enantiomers
d isotopes
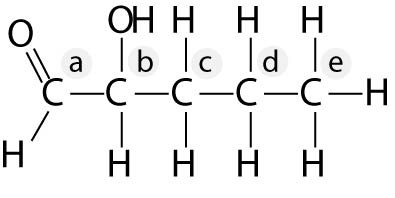
4-6
Identify the asymmetric carbon in this molecule
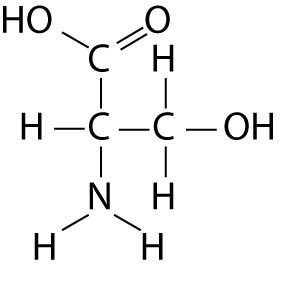
4-7
Which fuctional group is not present in this molecule?
a carboxyl
b sulfhydryl
c hydroxyl
d amino
4-8
Which action could produce a carbonyl group?
a the replacement of the hydroxyl of a carboxyl group with hydrogen
b the addition of a thiol to a hydroxyl
c the addition of a hydroxyl to a phosphate
d the replacement of the nitrogen of an amine with oxygen
e the addition of a sulfhydryl to a carboxyl
4-9
which functional group is most likely to be responsible for an organic molecule behaving as a base?
a hydroxyl
b carbonyl
c carboxyl
d amino
e phosphate
4-10
Given that you know about the electronegativity of oxygen predict which of the following molecules would be the stronger acid. Explain
5-1
Which term includes all others in the list?
a monosaccharide
b disaccharide
c starch
d carbohydrate
e polysaccharide
5-2
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by linking 10 glucose molecules together by dehydration reactions
a C60H120O60
B C6H12O6
C C60H102O51
D C60H100O50
E C60H111O51
5-3
The enzyme amylase can break glycosidic linkages between glucose monomers only if the monomers are the a form. Which of the following could amylase break down?
a cellulose
b chitin
c glycogen
d starch
e amylopectin
5-4
Choose the pair of terms that correctly completes this sentence: Nucleotides are to ___ as ___ are to proteins
a nucleic acids; amino acids
b amino acids; polypeptides
c glycosidic linkages; polypeptide linkages
d genes; enzymes
e polymers; polypeptides
5-5
Which of the following statements concerning unsaturated fats is true?
A They are more common in animals than in plants.
B They have double bonds in the carbon chains of their fatty acids.
C They generally solidify at room temperature.
D They contain more hydrogen than do saturated fats having the same number of carbon atoms.
E They have fewer fatty acid molecules per fat molecule.
5-6
The structural level of a protein least affected by a disruption in hydrogen bonding is the
A primary level.
B secondary level.
C tertiary level.
D quaternary level.
E All structural levels are equally affected.
5-7
Which of the following pairs of base sequences could form a short stretch of a normal double helix of DNA?
A 5'-purine-pyrimidine-purine-pyrimidine-3' with 3'-purine-pyrimidine-purine-pyrimidine-5'
B 5'-AGCT-3' with 5'-TCGA-3'
C 5'-GCGC-3' with 5'-TATA-3'
D 5'-ATGC-3' with 5'-GCAT-3'
E All of these pairs are correct.
5-8
Enzymes that break down DNA catalyze the hydrolysis of the covalent bonds that join nucleotides together. What would happen to DNA molecules treated with these enzymes?
A) The two strands of the double helix would separate.
B) The phosphodiester linkages of the polynucleotide backbone would be broken.
C) The purines would be separated from the deoxyribose sugars.
D) The pyrimidines would be separated from the deoxyribose sugars.
E) All bases would be separated from the deoxyribose sugars.
5-9
Which of the following is not a protein
a hemoglobin
b cholesterol
c an antibody
d an enzyme
e insulin
5-10
Which of the following statements about the 5' end of a polynucleotide strand of DNA is
correct?
A) The 5' end has a hydroxyl group attached to the number 5 carbon of ribose.
B) The 5' end hasa phosphate group attached to the number 5 carbon of ribose.
C) The 5' end has thymine attached to the number 5 carbon of ribose.
D) The 5' end has a carboxyl group attached to the number 5 carbon of ribose.
E) The 5' end is the fifth position on one of the nitrogenous bases.
6-1
The symptoms of a certain inherited disorder in humans include breathing problems and,
in males, sterility. Which of the following is a reasonable hypothesis for the molecular
basis of this disorder?
a. a defective enzyme in the mitochondria
b. defective actin molecules in cellular microfilaments
c. defective dynein molecules in cilia and flagella
d. abnormal hydrolytic enzymes in the lysosomes
e. defective ribosome assembly in the nucleolus
6-2
Which statement correctly characterizes bound ribosomes?
A) Bound ribosomes are enclosed in theirown membrane.
B) Bound and free ribosomes are structurally different.
C) Bound ribosomes generally synthesize membrane proteins and secretory proteins.
D) The most common location for bound ribosomes is the cytoplasmic surface of the plasmamembrane.
E) All of the above.
6-3
Which of the following is not considered to be a part of the endomembrane system?
a nuclear envelope
b chloroplast
c golgi apparatus
d plasma membrane
e ER
6-4
Cells of the pancreas will incorporate radioactively labeled amino acids into proteins. This "tagging" of newly synthesized proteins enables a researcher to track their location. In this case, we are tracking an enzyme secreted by pancreatic cells. What is its most likely pathway?
a ER→Golgi→nucleus
b Golgi→ER→lysosome
c nucleus→ER→Golgi
d ER→Golgi→vesicles that fuse with plasma membrane
e ER→lysosomes→vesicles that fuse with plasma membrane
6-5
Which of the following is present in a plant and animal cells?
a chloroplast
b wall made of cellulose
c tonoplast
d mitochondrion
e centriole
6-7
which type of cell would probably provide the best opportunity to study lysosomes?
a muscle cell
b nerve cell
c phagocytic white blood cell
d leaf cell of a plant
e bacterial cell
6-6
Which of the following is present in a prokaryotic cell
a mitochondrion
b ribosome
c nuclear envelope
d choloroplast
e ER
6-8
Which of the following statements is a correct distinction between prokaryotic and eukaryotic cells attributable to the absence of a prokaryotic cytoskeleton?
a organelles are found only in eukaryotic cells
b cytoplasmic streaming is not observed in prokaryotes
c only eukaryotic cells are capable of movement
d prokaryotic cells have cell walls
e only the eukaryotic cell concentration its genetic material in a region separate from the rest of the cell
6-9
which of the following structure-function pairs is mismatched?
a nucleolus; ribosome production
b lysosome; intracellular digestion
c ribosome;protein synthesis
d Golgi; protein trafficking
e microtubule; muscle contraction
6-10
Cyanide binds with at least one of the molecules involved in the production of ATP. Following exposure of a cell to cyanide. most of the cyanide could be expected to be found within the
a mitochondria
b ribosomes
c peroxisomes
d lysosomes
e endoplasmic reticulum
7-1
In what way do the membranes of a eukaryotic cell vary?
a Phospholipids are found only in certain membranes
b Certain proteins are unique to each membrane
c Only certain membranes of the cell are selectively permeable
d Only certain membranes are constructed from amphipathic molecules
e Some membranes have hydrophobic surfaces exposed to the cytoplasm, while others have hydrophilic surfaces facing the cytoplasm
7-2
According to the fluid mosaic model of membrane structure, proteins of the membrane are mostly
a spread in a continuous layer over the inner and outer surfaces of the membrane
b confined to the hydrophobic core of the membrane
c embedded in a lipid bilayer
d randomly oriented in the membrane, with no inside-outside polarity
e free to depart from the fluid membrane and dissolve in the surrounding solution
7-3
Which of the following factors would tend to increase membrane fluidity?
a a greater proportion of unsaturated phospholipids
b a greater proportion of saturated phospholipids
c a lower temperature
d a relatively high protein content in the membrane
e a greater proportion of relatively large glycolipids compared with lipids having smaller molecule masses
7-4
Which of the following processes includes all others?
a osmosis
b diffusion of a solute across a membrane
c facilitated diffusion
d passive transport
e transport of an ion down its electrochemical gradient
7-5
Based on the model of sucrose uptake in figure 7.19, which of the following experimental treatments would increase the rate of sucrose transport into the cell?
a decreasing extracellular sucrose concentration
b decreasing extracellular pH
c decreasing cytoplasmic pH
d adding an inhibitor that blocks the regeneration of ATP
e adding a substance that makes the membrane more permeable to hydrogen ions
7-6
Which solute will exhibit a net diffusion into the cell
7-7
Which solute will exhibit a net diffusion out of the cell
7-8
Is the solution outside the cell isotonic, hypotonic, or hypertonic?
7-9
In which direction will there be a net osmotic movement of water?
7-10
After the cell is placed in the beaker, which of the following changes will occur?
a the artificial cell will become more flaccid
b the artificial cell will become more turgid
c some water molecules will flow out of the cell but the majority will flow into it
d the membrane potential will decrease
e in spite of the inability of sucrose to cross the membrane, eventually the two solutions will have the same solute concentrations
10-1
The light reactions of photosynthesis supply the calvin cycle with
a light energy
b co2 and atp
c h20 and nadph
d atp and nadph
e sugar and o2
10-2
Which of the following sequences correctly represents the flow of electrons during photosynthesis?
A NADPH O2 CO2
B H2O NADPH Calvin cycle
C NADPH chlorophyll Calvin cycle
D H2O photosystem I photosystem II
E NADPH electron transport chain O2
10-3
Which of the following conclusions does not follow from studying the absorption spectrum for chlorophyll a and the action spectrum for photosynthesis?
a not all wavelengths are equally effective for photosynthesis
b the red and blue areas of the spectrum are most effective in driving photosynthesis.
c there must be accessory pigments that broaden the spectrum of light that contributes energy for photosynthesis
d chlorophyll owes its color to the absorption of green light
e chlorophyll a has two absorption peaks
10-4
Cooperation of the two photosystems is required for?
a ATP synthesis
b reduction of NADP
c cyclic photophosphorylation
d oxidation of the reaction center of photosystem 1
e generation of the proton-motive force
10-5
In mechanism, photophosphorylation is most similar to
A substrate-level phosphorylation in glycolysis.
B oxidative phosphorylation in cellular respiration.
C the Calvin cycle.
D carbon fixation.
E reduction of NADP+.
10-6
In what respect are the photosynthetic adaptations of c4 plants and cam plants similar?
a in both cases only photosystem I is used
b both types of plants make sugar without the calvin cycle
c in both cases an enzyme other than rubisco carries out the first step in carbon fixation
d both types of plants make most of their sugar in the dark
e neither c4 plants nor cam plants have thylakoids
10-7
Which process is most directly driven by light energy?
A creation of a pH gradient by pumping protons across the thylakoid membrane
B carbon fixation in the stroma
C reduction of NADP+molecules
D removal of electrons from chlorophyll molecules
E ATP synthesis
10-8
Which of the following statements is correct distinction between cyclic and noncyclic electron flow?
A In addition to ATP, cyclic electron flow also produces O2 and NADPH
B Only cyclic electron flow utlizes light at 700 nm.
C Only cyclic electron flow can operate in the absence of photosystem II.
D Only noncyclic electron flow produces ATP
E Chemiosmosis is unique to noncyclic electron flow
10-9
which of the following statements is a correct distinction between autotrophs and heterotrophs?
a only heterotrophs require chemical compounds from the environment
b cellular respiration is unique to heterotrophs
c only heterotrophs have mitochondria
d autotrophs, but not heterotrophs, can nourish themselves beginning with co2 and other nutrients that are inorganic
e only heterotrophs require oxygen
10-10
Which of the following does not occur during the calvin cycle
a carbon fixation
b oxidation of NADPH
c release of oxygen
d regeneration of the CO2 acceptor
e consumption of ATP
11-1
Phosphorylation cascades involving a series of protein kinases are useful for cellular signal transduction because
A they are species specific.
B they always lead to the same cellular response.
C they amplify the original signal manyfold.
D they counter the harmful effects of phosphatases.
E the number of molecules used is small and fixed.
11-2
Binding of a signal molecule to which type of receptor leads directly to a change in the distribution of anions and/or cations on opposite sides of the membrane?
a receptor tyrosine kinase
b G–protein–linked receptor
c phosphorylated receptor tyrosine kinase dimer
d ligand–gated ion channel
e intracellular receptor
11-3
The activation of receptor tyrosine kinases is characterized by
A dimerization and phosphorylation.
B IP3binding.
C a phosphorylation cascade.
D GTP hydrolysis.
E channel protein shape change.
11-4
which of the following provides the best evidence that cell signaling pathways evolved early in the history of life?
a they are seen in "primitive" cells such as yeast
b yeast cells signal each other for mating
c signal transduction molecules found in distantly related organisms are similar
d signals can be sent long distances by cells
e most signals are received by cell surface receptors
11-5
Which observation suggested to Sutherland the involvement of a second messenger in epinephrine′s effect on liver cells?
a Enzymatic activity was proportional to the amount of calcium added to a cell–free extract.
b Receptor studies indicated that epinephrine was a ligand.
c Glycogen breakdown was observed only when epinephrine was administered to intact cells.
d Glycogen breakdown was observed when epinephrine and glycogen phosphorylase were combined.
e Epinephrine was known to have different effects on different types of cells.
11-6
Protein phosphorylation is commonly involved with all of the following except
a regulation of transcription by extracellular signal molecules.
b enzyme activation.
c activation of G–protein–linked receptors.
d activation of receptor tyrosine kinases.
e activation of protein kinase molecules.
11-7
Amplification of a chemical signal occurs when
a a receptor in the plasma membrane activates several G– protein molecules while a signal molecule is bound to it.
b a cAMP molecule activates one protein kinase molecule before being converted to AMP.
c phosphorylase and phosphatase activities are balanced.
d receptor tyrosine kinases dimerize upon ligand binding.
e both a and d occur.
11-8
Lipid–soluble signal molecules, such as testosterone, cross the membranes of all cells but affect only target cells because
a only target cells retain the appropriate DNA segments.
b intracellular receptors are present only in target cells.
c most cells lack the Y chromosome required.
d only target cells possess the cytosolic enzymes that transduce the testosterone.
e only in target cells is testosterone able to initiate the phosphorylation cascade leading to activated transcription factor.
11-9
Signal transduction pathways benefit cells for all of the following reasons except
a they help cells respond to signal molecules that are too large or too polar to cross the plasma membrane.
b they enable different cells to respond appropriately to the same signal.
c they help cells use up phosphate generated by ATP breakdown.
d they can amplify a signal.
e variations in the signal transduction pathways can enhance response specificity.
11-10
Consider this pathway: epinephrine → G–protein–linked receptor → G protein → adenylyl cyclase → cAMP. Identify the second messenger.
a cAMP
b G protein
c GTP
d adenylyl cyclase
e G–protein–linked receptor
...