What is the octet rule?
The octet rule is a principle in chemistry that states atoms tend to gain, lose, or share electrons to achieve a total of eight valence electrons.
What are ionic bonds?
Ionic bonds form due to electrostatic attraction between positive and negative ions (cations and anions).
Why do metals become positive ions or cations?
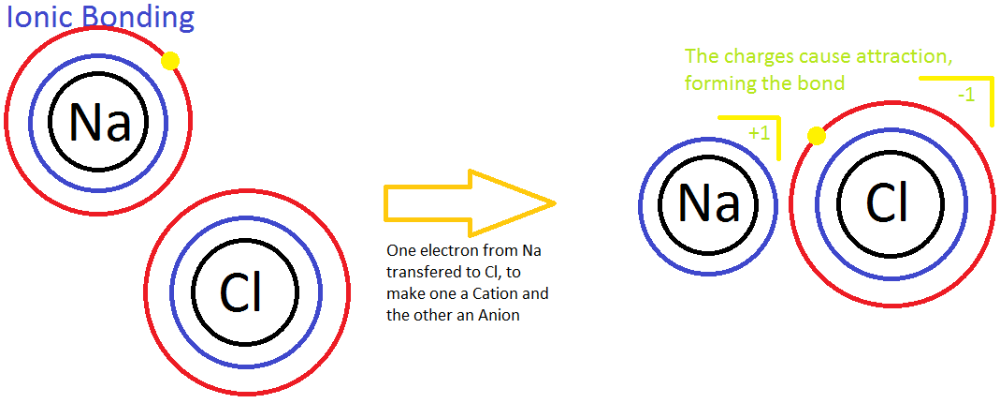
Metals have lower electronegativity therefore they become electron donors because it is easier for them to lose an electron rather than gain it, thus achieving their noble gas configuration becoming in the process positive ions or cations.
Why do non metals become negative ions or anions?

Non-metals have higher electronegativity making it easier for them to accept the electrons donated by the metals, adding this electrons to their valance shell helps non-metals to achieve their noble gas configuration by satisfying the octet rule becoming now negative ions or anions.
What is a formula unit?
The single unit of an ionic compound is called formula unit. The ratio of cations to anions in a formula unit is represented in the chemical formula.
What do ionic compounds get formed into?
Crystal lattice
What is the crystal lattice?
Ionic crystals are formed with several formula units arranged in the ratios of the single formula units. This arrangement is known as the crystal lattice, here the cations and anions are in direct contact and tightly bound, impeding further movement of electrons.
Why are ionic compounds neutral in solid form?
Electrons are locked in the lattice
Why are ionic compounds increase conductivity when in solution with water or molten?
Cations and Anions can move freely, permitting the flow of charge
Why are ionic compounds water soluble with some exceptions?
Due to water polarity and ionic charges
Why are ionic compounds have high melting points?
This will vary depending on the strength of the bond
Why are ionic compounds brittle?
Applied forces push formula units against each other, similar charges repel, which causes fractures in the lattice.
What determines the strength of ionic bonds?
a. Amount of charge: the higher the charge of the cations and anions the more energy is needed to break the bond i.e. MgS has a higher melting point than NaCl.
b. Ionic radii: When atoms have the same charges then we can look at the size of the ions. the smaller the distance between the nucleus of the ions the higher the energy required to break the force. I.e. NaCl melts at 800C vs NaBr which melts at 747C
What is covalent bonding?
Covalent bonds occur when atoms of non-metal elements share electrons between them. The sharing allows each atom to complete a full octet in their valance shell.
Do non metals in covalent bonds have similar negativites?
Non-metals that are involved in covalent compounds have similar electronegativities (higher than nonmetals) making it easier to share than to lose electrons, in this case both species usually would want to gain electrons, therefore sharing is an easy way to achieve stability.
What is the basic unit of a covalent bond?
The molecule
Why are covalent bonds poor conductors of electricity?
Shared electrons locked in place, no flow. Molecules are not charged, so even when dissolved in water they are not good conductors.
Why are covalent bonds if solids usually waxy?
Weak intermolecular forces allow molecules to slip past each other
Why are most covalent bonds in liquid or gas states?
Extremely weak intermolecular forces
Why are most covalent compounds are insoluble in water?
Polar vs non-polar molecules (basically partially + and - charged molecules)
Why are covalent bonds sometimes able to dissolve?
...