Describe how the scattering of alpha (α) particles by a sheet of thin metal supports the nuclear model of the atom
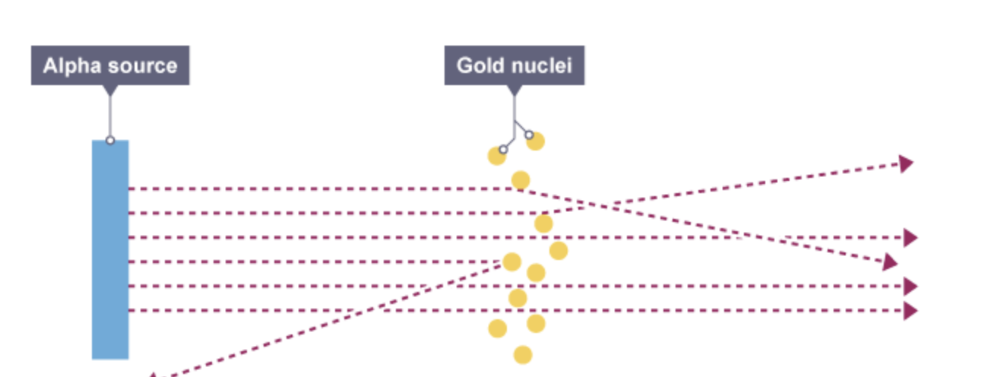
- the fact that most alpha particles went straight through the foil is evidence for the atom being mostly empty space
- a small number of alpha particles being deflected at large angles suggested that there is a concentration of positive charge in the atom - like charges repel, so the positive alpha particles were being repelled by positive charges
- the very small number of alpha particles coming straight back suggested that the positive charge and mass are concentrated in a tiny volume in the atom (the nucleus) - the tiny number doing this means the chance of being on that exact collision course was very small, and so the 'target' being aimed at had to be equally tiny
(a) a very small nucleus surrounded by mostly empty space
(b) a nucleus containing most of the mass of the atom
(c) a nucleus that is positively charged
Describe the processes of nuclear fission and nuclear fusion and description of mass and energy changes
Nuclear fission is the splitting of a large atomic nucleus into two or more smaller nuclei, releasing energy.
Nuclear fusion is the joining of two or more light nuclei to form a heavier nucleus, releasing even more energy.
nuclear fission and nuclide equation
A heavy nucleus (like Uranium) absorbs a neutron and becomes unstable. This unstable nucleus then splits into two or more lighter nuclei, along with several neutrons and a large amount of energy.
235 92U + 1 0n = 92 36Ba + 141 56Kr + 3 0n Balanced equation. n is neutron
nuclear fusion and nuclide equation
Two or more light nuclei (like Hydrogen) collide at very high speeds and fuse together, forming a single, heavier nucleus.
²H + ³H → ⁴He + ¹n Balanced equation. n is neutron
Equation to calculate energy created in nuclear fission and nuclear fusion
In both processes, a small amount of mass is converted into a large amount of energy, following Einstein's equation E=mc², where E is energy, m is mass and c is speed of light (300,000 kilometers per second).
x yZ define x and y
proton number (atomic number) and nucleon number (mass number)
Define isotope and why radioactive
different atoms of the same element that contain the same number of protons but a different number of neutrons
radioactive due to an excess of neutrons in the nucleus and/or the nucleus being too heavy
Define background radiation and sources 4
Low level nuclear radiation that is always present from natural and man-made sources.
(a) radon gas (in the air)
(b) rocks and buildings
(c) food and drink
(d) cosmic rays
How to measure radiation
Ionising nuclear radiation can be measured using a detector connected to a counter.
Count rate measured in counts / sec or counts / minute. The count rate indicates the number of radioactive decays occurring per unit of time.
To find corrected count rate first measure background radiation without the radiation source. then measure with source and subtract background radiation count.
Describe radioactive decay
radioactive decay is a change in an unstable nucleus that can result in the emission of α-particles or β-particles and/or γ-radiation and these changes are spontaneous and random.
What alpha (α), beta (β) and gamma (γ) are
- Alpha (α):An alpha particle is a helium nucleus consisting of two protons and two neutrons. It is a positively charged particle.
- Beta (β):A beta particle is an electron (β-) emitted from the nucleus.
- Gamma (γ): Gamma radiation is a high-energy form of electromagnetic radiation, similar to X-rays but with much higher energy. Gamma rays are uncharged particles.
How alpha (α), beta (β) and gamma (γ) are effected my charge and magnetic fields, ionising power and penetrating.
A higher kinetic energy generally leads to a greater ionising effect (a higher mass results in higher kinetic energy). The higher the charge, the greater the ionising effect.
- Alpha:Alpha particles have the highest ionising power. Due to their large size and charge (+2), they interact strongly with matter, causing significant ionisation along their path. Therefore least penetrating (stopped by paper)
- Beta: Beta particles are next. with -1 charge and smaller mass. Their smaller size allows them to penetrate deeper into matter before interacting with atoms. (stopped by thin metal sheet)
- Gamma:Gamma rays have the lowest ionizing power. Because they are electromagnetic radiation, they interact less with matter and are more likely to pass through it as not deflected as no charge and virtually no mass (MOST penetrating stopped by thick layer of metal or concrete).
- Alpha particles: Being positively charged, they are deflected towards the negative plate. Their deflection is less than beta particles because they are much heavier. Also deflected in magnetic fields as charged.
- Beta particles: Being negatively charged, they are deflected towards the positive plate. They are deflected more than alpha particles due to their lighter mass. Also deflected in magnetic field but opposite direction of alpha as negative.
- Gamma rays: As they are electrically neutral, they are not deflected and continue in a straight path. Also not affected by magnetic fields.
nuclide notation, to show the emission of α-particles, β-particles and γ-radiation and effect on stability
Gamma rays, which are high-energy electromagnetic radiation, are emitted so lead to a lower, more stable energy state without altering its composition. (A ZX* = A ZX + 0 0y where x* is the excited nucleus.
Alpha rays lose 2 protons and 2 neutrons leading to the mass number decreasing by 4 and the atomic by 2. This makes it more stable by reducing its size. (A ZX = A-4 Z-2X + 4 2a
During beta decay, a neutron within the nucleus is converted into a proton and an electron (neutron → proton + electron), with the electron being emitted as a beta particle. This process leads to an increase in the atomic number by 1, while the mass number remains unchanged. The stability of the nucleus is increased by reducing the number of excess neutrons and achieving a more favorable neutron-to- proton ratio. (A ZX = (A Z+1X + 0 -1B)
Define the half-life of a particular isotope
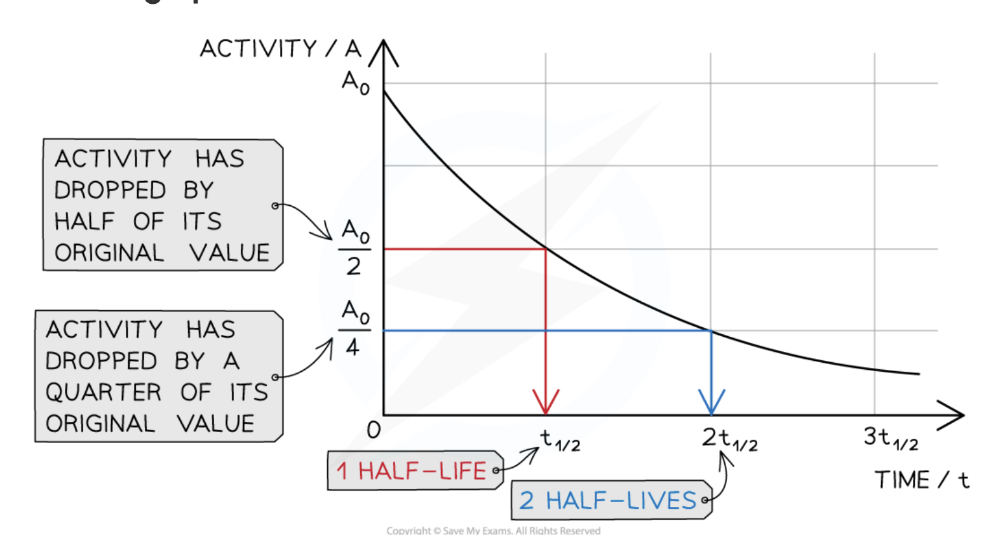
The time taken for half the nuclei of that isotope in any sample to decay.
- The half-life of an isotope should be calculated by removing the background radiation from data or decay curves
Explain how the type of radiation emitted and the half-life of an isotope determine which isotope is used for applications including:
(a) household fire (smoke) alarms
(b) irradiating food to kill bacteria
(c) sterilisation of equipment
(d) measuring and controlling thicknesses of materials with the choice of radiations used
linked to penetration and absorption
(e) diagnosis and treatment of cancer using
gamma rays
- Household fire (smoke) alarms: use an alpha emitter because alpha radiation is highly ionizing and easily absorbed by smoke particles, triggering the alarm when smoke interrupts the radiation flow between two electrodes. Additionally, a relatively long half-life is needed to ensure the alarm doesn't need frequent battery replacements.
- Irradiating food to kill bacteria: uses gamma radiation from isotopes because gamma rays are highly penetrating and can reach deep into food products to kill bacteria effectively. A longer half-life is desirable for consistent radiation output during the irradiation process.
- Sterilisation of equipment using gamma rays: gamma rays are used for sterilisation due to their high penetration. Again, a suitable isotope with a long half-life is chosen for consistent radiation delivery.
- Measuring and controlling thicknesses of materials: Beta radiation is ideal because it can penetrate thin materials like metal sheets but is easily absorbed by thicker materials. A moderate half-life ensures stable measurements over time.
- Diagnosis and treatment of cancer using gamma rays: For diagnostic imaging, short-lived isotopes are often preferred to minimise patient exposure while providing sufficient imaging information. For cancer treatment, longer half-life gamma emitters are used to deliver a concentrated dose of radiation to the tumor area. Specific isotopes chosen depend on the type of cancer and treatment needs.
State the effects of ionising nuclear radiations on living things (3)
Causes cell death by directly destroying cell structures and DNA, leading to the cell's death or malfunction. It also causes mutations by damaging DNA, which can result in uncontrolled cell division and cancer when the mutated cells reproduce. Cancer is a result of mutations that cause cells to divide and multiply in an uncontrolled manner.
Explain safety precautions for all ionising radiation
Moving and Using and storing Radioactive Materials
- Containment : Radioactive materials must be kept in shielded containers, such as lead-lined boxes, when not in use.
- Remote Handling:Use remote tools like tongs or robotic arms to handle materials from a distance, increasing safety.
- Personal Protective Equipment (PPE):Wear gloves, lab coats, and safety glasses to prevent contamination.
- Limit Exposure:Minimize the amount of time spent near the radioactive source to reduce the total radiation dose received.
- Access Control: Restrict access to areas where radioactive materials are used to ensure only trained personnel are present and to maintain distance from the source.
- Labelling : Clearly label all containers with the isotope and its activity level.
- Waste Disposal : Dispose of radioactive waste according to specific regulations for different types of radiation, using appropriate canisters and storage facilities.
Safety Precautions for Ionising Radiation
- Reduce Exposure Time: The less time you spend near a radioactive source, the less radiation you will be exposed to.
- Increase Distance: Radiation intensity decreases rapidly with distance, so keeping as far away from the source as possible significantly reduces your exposure.
- Use Shielding: Place absorbing materials between yourself and the radioactive source to block the radiation.