what is a teratogen
A teratogen is a substance that can interfere with normal fetal development and cause congenital disabilities1. Examples of teratogens include drugs, alcohol, chemicals, certain infections, and toxic substances1.
Teratogens can cause abnormalities in a developing embryo or fetus when a person is exposed to or ingests them during pregnancy1. The risk of damage from teratogen exposure during pregnancy depends on several factors, including the type of toxin, the duration of exposure, the amount of exposure, the gestational age of the fetus at exposure, and hereditary factors
Know the germ layers and what they become
Germ layers formed
Ectoderm→ skin and nervous
system
Mesoderm→ bone, muscle, connective
tissue
Endoderm→ linings of digestive and
respiratory
system and other organs
Know basic order of embryogenesis
Embryogenesis is a complex process that transforms a single cell into a fully formed organism. Here are the basic stages of embryogenesis:
- Fertilization: A sperm cell fuses with an egg cell to form a single cell called a zygote1 2.
- Cleavage: The zygote undergoes rapid cell division, doubling in cell number with each round of division1. This stage results in a 32-cell structure known as a morula1.
- Blastulation: The morula develops into a hollow ball of cells called a blastocyst1 3. The blastocyst consists of an outer layer of cells (the trophoblast), an inner cell mass (the embryoblast), and a fluid-filled cavity (the blastocoel)1.
- Implantation: The blastocyst implants in the uterus4.
- Gastrulation: The blastocyst develops into a gastrula, which has three germ layers: the ectoderm, mesoderm, and endoderm3.
- Organogenesis: The germ layers develop into different organs and tissues3.
- Growth and Differentiation: The embryo continues to grow and the cells continue to differentiate into more specific cell types2.
list a couple mechanism for teratogenesis
mutations, chromosomal breaks, altered mitosis, altered
nucleic
acid integrity or function, diminished supplies of precursors
or
substrates, decreased energy supplies, altered membranes,
osmolar imbalance, enzyme inhibition, etc
Know what the placenta is and what affects a drugs ability to pass
The placenta
• The placenta plays a central role in transferring
nutrients,
important hormones/peptides, and waste products
between
the conceptus and mother
• The extent of the
transfer depends on three major factors
• type of
placentation
• physicochemical properties of the chemical
•
rates of placental metabolism
• Most drug passage across the
placenta seems to occur by
simple passive diffusion
•
Lipid solubility, molecular weight, ionization
• Placenta
toxicity can influence the metabolic “programming of
the
fetus”
•Note: other maternal toxicity responses can affect the
developing fetus
The placenta is a temporary organ that develops in the uterus during pregnancy1 2. It attaches to the wall of the uterus, and the baby’s umbilical cord arises from it1. The placenta provides oxygen and nutrients to the growing baby and also removes waste products from the baby’s blood1. It plays a significant role in fetal and adult health, and placental pathology is implicated in all common obstetric complications3.
The ability of a drug to pass through the placenta and reach the fetus is influenced by several factors4 3 5 6 7:
- Molecular weight: Drugs with a low molecular weight can more easily cross the placental barrier5 6 7.
- Lipid solubility: Drugs that are lipid-soluble can more readily diffuse across cell membranes, including the placental barrier4 6 7.
- Degree of ionization: Non-ionized drugs can more easily cross the placenta4 5.
- Protein binding: Drugs that are bound to proteins in the mother’s blood are less likely to cross the placenta7.
- Concentration gradient: A higher concentration of a drug on the maternal side of the placenta compared to the fetal side can drive diffusion of the drug across the placenta4 5.
- Placental blood flow: The volume of blood perfusing the placenta can affect the rate of drug transfer4 5.
- Placental membrane characteristics: The physical properties of the placental membrane, including its surface area and thickness, can affect drug transfer4 5.
- Pathological changes in the placenta: Any pathological changes in the placenta can affect its ability to act as a barrier4 5.
Be able to give a couple of examples of the air pollutants of major
concern to
human health
Air pollutants of major public health concern
•Particulate
matter (PM)
•Carbon monoxide (CO)
•Nitrogen dioxide
(NOX)
•Sulfur dioxide (SOX)
•Metals & metalloids (like
lead)
•Green house gases (CO2)
•Hydrocarbons
•Volatile
Organic Compounds (VOCs)
•Ozone (O3).
Know some of the major sources of air pollutants
Anthropogenic Sources of Air Pollution:
• Fuel combustion e.g.
motor vehicles
• Heat and power generation (e.g. oil and coal
power plants and boilers)
• Industrial facilities (e.g.
manufacturing factories, mines, and oil refineries)
•
Residential cooking, heating, and lighting with polluting fuels
• Municipal and agricultural waste sites and waste
incineration/burning
• Forest fires
• Natural sources
• Volcanoes
• Wildfires
• Windblown dust
•
Natural biogenic vapors
Define particulate matter and know the size limits for the classifications of PM
EPA definition of Particulate Matter (PM): term
for a mixture
of solid particles and liquid droplets
found in the air. Some
particles, such as dust, dirt,
soot, or smoke, are large or dark
enough to be
seen with the naked eye. Others are so small they
can only be detected using an electron
microscope.
•
PM10 : inhalable particles, with diameters that
are generally 10
micrometers and smaller; and
• PM2.5 : fine inhalable particles,
with diameters
that are generally 2.5 micrometers and smaller.
Understand where we want and don’t want ozone, and the sources of each
•Ozone, at concentrations that occur in urban areas, induces in
humans and experimental animals morphologic, functional,
immunologic, and biochemical alterations mostly in the
lungs.
• O3 can irritate the lining of the nose, airways and
lungs.
• Exposure to O3 can produce a variety of pulmonary
function
changes
• i.e. chest pain and discomfort with
breathing, pulmonary edema
• People with asthma might have more
attacks and athletes might find it harder
to perform as well as usual.
• About 10 to 50 km above the Earth’s surface, UV light directly
splits
molecular O2
into atomic O•, which then combines
with O2
to form
O3
.
• Ozone layer: thin
“permanent” barrier that absorbs
the short-wavelength UV light
Know some general sources of water pollution
Some sources of water pollution
◦ Sewage (waste water)
◦
Agricultural Pollution
◦ Oil/gasoline Pollution
◦
Radioactive Substances
◦ River dumping
◦ Industrial (e.g.
mining, smelting)
◦ Pharmaceuticals & Personal Care Products
(PPCPs)
◦ Water Disinfection By-Products
Know potential consequences of drinking water contaminated with nitrate
Consuming too much nitrate can
affect how blood carries oxygen
and
can cause methemoglobinemia (blue
baby syndrome).
• Other symptoms connected to
methemoglobinemia include
decreased blood pressure, increased
heart rate, headaches,
stomach
cramps, and vomiting.
Understand how Flint water was contaminated with lead during the
Flint
water crisis
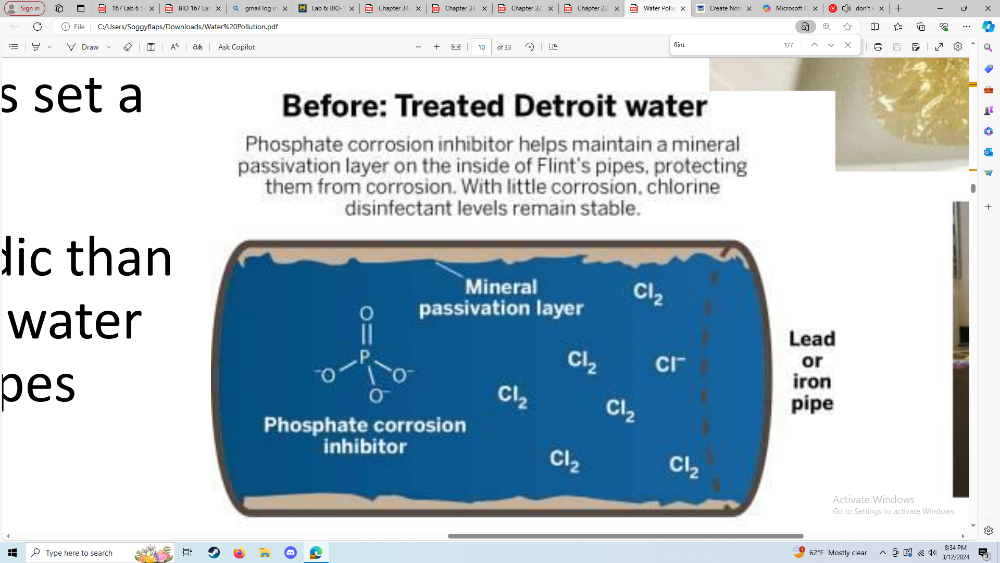
Financial issues caused the state to decide to
cut back on the
cost of water in Flint by using
local sources instead of buying
water from
Detroit
• Lead levels were high: 15-30 μg/L,
some
reports much higher than that
•. NO levels of lead are
safe. The EPA has set a
max level (action level) of 15 μg.
• Flint River water was slightly more acidic than
the
purchased water from Detroit. The water
ate away the protective
layers in the pipes
• Adding phosphates
Understand why some water is fluoridated and a potential harm
Pro: increasing health equity in
dental health
Con: Developmental neurotoxicity
Water is fluoridated to reduce tooth decay. Fluoride is a mineral that can strengthen tooth enamel, making it more resistant to decay1 2. Many towns and cities add fluoride to their water supply because most water doesn’t have enough natural fluoride to prevent tooth decay3. The process of adjusting the amount of fluoride in a public water supply to a level known to make teeth stronger and more resistant to cavities is called water fluoridation2.
However, there are potential harms associated with water fluoridation. Excessive fluoride can cause fluorosis, which is a defect in tooth enamel that ranges from barely noticeable white spots to staining and pitting4. Fluoride can also become concentrated in bone, stimulating bone cell growth, altering the tissue’s structure, and potentially weakening the skeleton4 5. Moreover, there is a modest body of evidence suggesting that fluoride, at doses considerably higher than what’s generally in the water, might be harmful to human brains, particularly developing fetal brains6.
It’s important to note that the potential risks from consuming fluoridated water may outweigh the benefits for some individuals, especially in an era of fluoridated toothpastes and other consumer products that boost dental health4. Therefore, the use of fluoride should always be discussed with a healthcare provider to weigh the potential benefits and risks.
Know what persistent organic pollutants are
A group of chemicals that have been and
continue to be of
significant environmental
concern
• Persistent in the
environment
• Prone to long-range, global transport
•
Bioaccumulate through the food web
• Toxic to living
organism
• Associated adverse effects: disruption of
the
endocrine, reproductive, and immune
systems, and
potential ability to cause
behavioral problems, cancer, diabetes,
and
thyroid problems.
Distinguish between different types of pesticides (e.g. insecticides
vs herbicides)
Pesticides: any substance or mixture of substances
intended for preventing,
destroying, repelling, or mitigating
pests.
◦ Insecticides: insects
◦ Herbicides: weeds
◦
Fungicides: fungi and molds
◦ Rodenticides: rodents
Often
formulations: active ingredient + other compounds to allow for mixing,
dilution, application, or stability
Herbicides
•Chemicals capable of killing or
severely injuring plants
• Preplanting herbicides: applied to
the soil before the crop is seeded
• Preemergent herbicides:
applied to soil before the time of appearance of unwanted
vegestation
• Postemergent herbicides: applied to the soil
after the germination of crop and/or weeds
• Contact herbicides:
those that affect the plant that was treated
• Translocated
herbicides: applied to the soil or above-ground parts of the plant
that are
absorbed and circulated to distant tissues
•
Nonselective herbicides: kill all vegetation
• Selective
herbicides: kill weeds without harming the crops
Insecticides
•All chemical insecticides used
today are
neurotoxicants
•As a class, insecticides have a
higher acute
toxicity towards nontarget species compared to
other pesticides
Know how DDT kills insects (and causes human toxicity) and why it
is banned in some countries but not others
DDT and Malaria
• DDT
(Dichlorodiphenyltrichloroethane): an organochlorine
insecticide
• DDT was introduced in 1942 as an insecticide
• DDT was banned in most countries by the mid 1970s because of
adverse human
health and environmental effects
• It was
banned in South Africa in 1996 (at the time ~10,000 cases of malaria
registered)
• By 2000, malaria cases reached 62,000
•
After the reintroduction of DDT at the end of the year, the cases of
malaria
were down to 12,500
DDT mechanism of action and toxicity
• Both in
insects and in mammals, DDT interferes with the sodium channels in the
axonal membrane
• Acute exposure to high doses of DDT
causes motor unrest, increased frequency
of spontaneous
movements, abnormal susceptibility to fear, and
hypersusceptibility to external stimuli (light, touch, sound).
• Chronic exposure to DDT targets the liver: increase liver
weight, cause hepatic
cell hypertrophy and necrosis, and potent
inducers of CYPs. It also has been
shown to be
endocrine-disrupting, have reproductive effects in both males and
females, and is classified as a probable carcinogen
Know why Bacillus theringiensis is specific to killing insects
Bacillus Thuringiensis
• Bacillus thuringiensis
(Bt) is a soil microorganism that forms spores containing
protein crystals
• When insects eat the spores, toxins are
proteolytically activated in the midgut
• The toxins create pores
in epithelial cells, ultimately destroying them. The
insects die
of gut paralysis
• The selective toxicity of Bt is attributed to
the fact that crystalline Bt endotoxins
require activation by
alkalis and/or digestion, conditions absent in the
mammalian
stomach.
•Bt toxins have generally an unremarkable toxicological
profile in mammals
Know how glyphosate is specific to killing plants
• Glyphosate (N-phosphonomethyl glycine): one of
the most
widely used herbicide in the world
• Inhibits
5-enolpyruvylshikimate-3-phosphate synthase,
which is
responsible for the synthesis of an
intermediate in the
biosynthesis of various amino acids
important in plant growth
(not present in mammals)
Know a historical fact about the history of forensic toxicology
Historical forensic toxicology
• Charles Norris was New York’s
first appointed chief medical
examiner and Alexander Gettler was
the head of his head
toxicologist
• The work of Norris and
Gettler ultimately lead to the
widespread acceptance of science
in crime investigations
• Arsenic, methanol, cyanide, morphine,
lead, mercury, carbon
monoxide, radium
Know general steps in a toxicology investigation
The toxicological investigation of a poison death may be divided into
three steps:
(1) obtaining the case history and suitable specimens,
(2)
the toxicological analyses, and
(3) the interpretation of the
analytical findings.
Know examples of specimens that are used for investigation
Specimens of many different body fluids and organs are collected
(before embalming)
• Blood, urine, liver tissue, stomach
contents
• Bone marrow, hair, nails, skeletal remains, vitreous
humor of the eye, saliva,
sweat, amniotic fluid, breast milk, semen
Describe techniques used in toxicological analysis
For oral administration of poison
• GI content
analysis: there may be large amounts of residual
poisons present
• Urine analysis: the kidney is a major organ of excretion for
most
poisons
• Liver: after absorption from the GI track,
things are carried to the
liver before entering systemic circulation
Initially: non-specific tests designed to determine
the presence
or absence of a class or group
• Urine tests
(like FPN color test)
• Examples of techniques used to isolate
and identify the
compound
• Gas chromatography (GC)
•
LC-MS
• High-resolution MS (TOP and Orbitrap)
• Biotransformation of the poison and normal chemical changes
that occur during the decomposition of a cadaver must be
considered
• During decomposition
• Phenylalanine →
phenylethylamine (chemical and physical
properties similar to
amphetamine)
• Hydrolysis/oxidation/reduction of
proteins/lipids/nucleic acids
generate numerous compounds that
may interfere with analysis
Know the role of toxicology in living criminal cases
Forensic toxicologist are becoming more involved in the
analysis of specimens from living victims
• Administration
of drugs to incapacitate→ kidnapping, robbery,
sexual assault
• benzodiazepines (Rohypnol), phenothiazines
• Poisoning
as a form of child abuse
• Laxatives, diuretics, table salt,
narcotics, antidepressants, sedative narcotics
Know the basic strategy for treatment of a poisoned patient
1. Clinical stabilization of the patient
2. Clinical evaluation
(history, physical, laboratory, and radiology)
3. Prevention of
further toxicant absorption
4. Enhancement of toxicant
elimination
5. Administration of antidote (if available)
6.
Supportive care, close monitoring, and clinical follow-up
Understand what the use of anion and osmol gaps are in poison diagnostics
• Because of the limited clinical availability
of rapidly
available “diagnostic” laboratory
tests for poisons, anion gap
and osmol gap
calculations may be helpful diagnostic
aids
(though measurements should be
interpreted cautiously)
• The AG is calculated as the difference
between the serum
Na ion concentration and
the sum of the serum Cl and
HCO3
ion concentrations. A normal AG is <12.
The osmol gap is calculated as the
difference between the
measured serum
osmolality and the serum osmolarity
calculated from the clinical chemistry
measurements of the
serum sodium ion,
glucose, and blood urea nitrogen (BUN)
concentrations. The normal osmol gap is <
10 mOsm.
Know potential strategies to prevent further poison absorption
Prevention of further poison absorption
•
Preventing further absorption of a poison may be possible
if the
exposure is oral, inhalation, or topical
•Inhalation: remove
patient from exposure site into clean air with
proper
ventilation
• Topical: remove contaminated clothing, gently wash
skin with mild
soap (be careful not to cause abrasions that
could enhance
absorption!)
•Oral (most effective ASAP):
induction of vomiting, gastric lavage,
oral administration of
activated charcoal, whole-bowel irrigation
Know potential strategies to enhance poison elimination
Alkalinization of the urine: increase urinary filtrate pH to ionize
weak acids
• Hemodialysis
• Hemoperfusion
•
Hemofiltration
• Plasma exchange or exchange transfusion
•
Administration of activated charcoal serially
Understand why there are a fairly small number of specific antidotes available
Use of antidotes in poisoning
• There are a relatively small
number of specific antidotes
available for clinical use
•
Practical difficulties in performing clinical research in poisoned
patients
• Small financial incentive for commercial
development
• The mechanisms of action of antidotes vary
•
Physically bind to toxicant, preventing deleterious effect (e.g.,
chelating
agent for heavy metals)
• Opposite effect on a
receptor (e.g., atropine vs organophosphate
insecticides)
• Chemical reaction that induces detoxifying capacity (e.g.,
sodium nitrate
and cyanide)
Know how sodium nitrate counters cyanide poisoning
Antidote example: sodium nitrate and cyanide
• Cyanide binds to
the ferric ions in cytochrome c oxidase
• When sodium nitrate is
given to someone with cyanide poisoning, it
induces the
formation of methemoglobin, which serves as an
alternative
binding site for the cyanide ion. Cyanomethemoglobin is
then
broken down in the body.
• Sodium thiosulfate acts as a sulfur
donor in the conversion of
cyanide to thiocyanate, which is
poorly retained in the mitochondria.
Understand some of the methods used to mitigate risks of hazardous
substances in the workplace
Occupational hierarchy for risk prevention
1. Substitution
where hazardous chemical or activities are removed and
substituted with a safer chemical or process
2. Engineering
controls that focus on reducing workplace exposures
3.
Administrative controls that can be used to change job tasks or
methods
4. Use of PPE
Be able to give an example of a disease associated with a specific
occupation
(and substance)
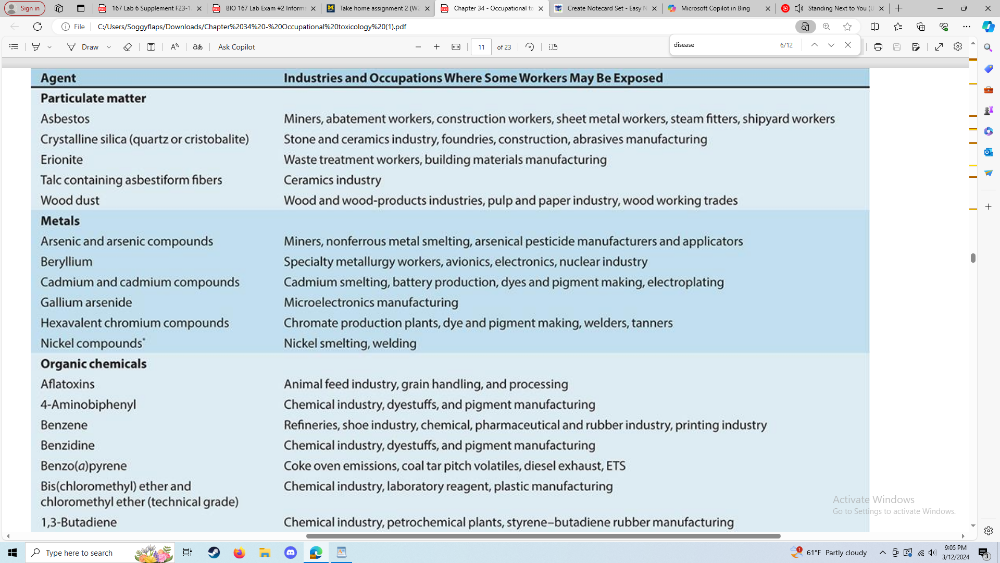
Occupational Exposure Agents
Classified by IARC as Group 1
Definite Human Carcinogens
Understand the testing that is involved in assessing occupational
risks and the
benefits/limitations of each of the following
types of studies: in vitro assays,
animal toxicity studies,
human challenge studies, case reports, and
epidemiology studies
Evaluation of occupational risks
•In vitro
assays
• Mechanistic insight
• Animal toxicology
studies
• Adverse effect, mechanistic data, dose-response
relationship
• Human challenge studies
• Verify animal
toxicity studies, biotransformation pathways, rates of uptake and
excretion,
threshold concentrations for response.
•
Benefits balanced against risks
• Case reports
• Workplace
surveillance or worker reports. These may lead animal or epidemiologic
studies
• Epidemiology studies
• Unravel associations
between occupational diseases, exposures, and personal risk factors
Know where the protective ozone layer is and how it is formed
the protective ozone layer is in the earth's atmosphere at an altitude of about 6.2 miles. Free oxygen atoms will collide with one another and bind together
Know the basic mechanism of carbon monoxide poisoning
carbon monoxide will build up in blood. This forms carboxyhemoglobin (COhb). CO2 has a high affinity for red blood cells. When COhb increases carrying capacity decreases then tissue receives less O2
Know the basic mechanism/disease caused by nitrate poisoning
this can affect how blood carries oxygen and can cause methemoglobimia (blue baby syndrome)
can be taken up by food, medications, home products
the nitrate is converted to nitrite then it reacts with hemoglobin
Know the basic techniques used in forensic technology (Mass spec, Gas chromatography, liquid chromatography, breathalyzer)
Mass spec =mass to charge ration of ion. identifies/quantifies compounds based on unique mass spec. Liquid mass spec, gas mass spec (examples of this include ion exchange chromatography)
Gas chromatography = Vaporizes samples and passes then through a column with a carrier gas to sperate based on volatility. separates volatile and low weight compounds
liquid chromatography = dissolved un a liquid phase based on their interaction with the column's stationary phase. Nonvolatile substances
breathalyzer =measure blood alcohol concertation (ethanol)
What are the benefits/limitations of in vitro studies for occupational toxicology
Benefits = mechanistic insight, animal toxicology studies, does-response relations ship
limitations = not exactly human testing, overlooking of systematic effects, technical limitations
What are the benefits/limitations of human challenge studies for occupational toxicology
Benefits = verify animal toxicology studies, biotransformation pathways
limitations = ethical considerations
Describe why we add phosphates to drinking water
to prevent the release of metals in drinking water such as lead and copper
polyphosphates sequester iron and magnesium to prevent discolored water
reduces exposure to lead
-
Orthophosphate Treatment:
- Purpose: Water systems add orthophosphate to drinking water to prevent lead pipes from leaching.
- Reaction: When orthophosphate is added to the water, it reacts with lead to form a mineral-like crust inside lead pipes.
- Protective Coating: This crust acts as a protective coating, preventing further lead corrosion and minimizing the release of lead into the water1 2.
-
Solid Lead Phosphate Formation:
- Mechanism: Orthophosphate reacts with lead and copper to create compounds that have a strong tendency to remain in solid form.
- Stability: These lead phosphate compounds adhere to the pipe walls, reducing the dissolution of lead into the water.
- Factors: The effectiveness of orthophosphate depends on its concentration, pH, dissolved inorganic carbon (DIC), and the characteristics of existing corrosion scale (e.g., presence of other metals like iron or aluminum)2.
Describe the mechanism for toxicity for organophosphates
Organophosphate poisoning occurs after exposure to organophosphates (OPs), which are used as insecticides, medications, and nerve agents. Let’s delve into the mechanism of toxicity:
- Inhibition of Acetylcholinesterase (AChE):
- Key Mechanism: Organophosphates irreversibly inhibit the enzyme acetylcholinesterase (AChE).
- Normal Function of AChE: AChE breaks down the neurotransmitter acetylcholine (ACh) at cholinergic synapses.
- Consequence: Inhibition of AChE leads to accumulation of ACh in the body1 2.
- Result: Continuous stimulation of nicotinic and muscarinic acetylcholine receptors.
- Cholinergic Crisis:
-
Central Nervous System Effects:
- Overstimulation of nicotinic receptors: Anxiety, headache, convulsions, ataxia, and tremors.
- Muscarinic overstimulation: Visual disturbances, chest tightness, wheezing, increased secretions, salivation, lacrimation, sweating, and urination.
-
Respiratory and Circulatory Depression:
- Depressed respiration and circulation.
- Potential coma and death3.
-
Central Nervous System Effects:
- Status Epilepticus (SE):
- Acute Exposure: Surge of ACh in cholinergic synapses.
- Result: Peripheral cholinergic crisis or SE.
- Severity: Can be lethal4.
- Treatment:
- Atropine: Blocks muscarinic receptors to counteract cholinergic effects.
- Oximes (e.g., pralidoxime): Reactivate inhibited AChE.
- Diazepam: Manages seizures.
- General Measures: Oxygen, intravenous fluids, and supportive care1 2.
Understand the pros and cons of not banning DTT
Pros: stops malaria, is cheap
cons, can cause birth defects and other health issues such as cancer and abnormal childhood brain development
What is the mechanism of cyanide poising and how is it treated
Cyanide disrupts cellar reparation by binding to cytochrome oxidase blocking intracellular reparation and increase lactic acid synthesis
treated by: Hydroxocobalamin in bind to or detoxify cyanide. can also use sodium nitrate.
Give an example of a disease (or symptoms) associated with a specific occupation/toxic substance
Lung cancer when exposed to asbestos in a manufacturing building. This can cause asthma or COPD before the cancer is formed or caught.