what is the location of fatty acid oxidation?
Fatty acid oxidation primarily takes place in the mitochondria of heart, skeletal muscle, and liver cells.
What is the location of fatty acid elongation?
Fatty acid elongation occurs in three cellular compartments: the cytosol, mitochondria, and endoplasmic reticulum (ER).
Cytosol:
- In the cytosol, fatty acid elongation involves the addition of carbon atoms to existing fatty acids.
- The process uses malonyl-CoA as a chain extender.
- The resulting elongated fatty acids contribute to various cellular functions.
Mitochondria:
- Mitochondria can also participate in fatty acid elongation.
- However, the starting materials for mitochondrial elongation are generally shorter than 16 carbons.
- Mitochondrial elongation is less common compared to other compartments.
Endoplasmic reticulum:
- The ER is a major site for fatty acid elongation.
- Using the same set of enzymes as other fatty acid synthesis steps, the ER elongates fatty acids.
- Unlike cytoplasmic synthesis, the elongation process in the ER is separate and not part of a complex.
- Propionyl-CoA is used instead of acetyl-CoA for elongation beyond 16 carbon-chain fatty acids.
What is the location of fatty acid synthesis?
Fatty acid synthesis occurs in the cytoplasm of the cell.
What is the location of fatty acid desaturation?
Fatty acid desaturation occurs in the endoplasmic reticulum (ER) of the cell.
What is the location of sterol synthesis (cholesterol)?
Cholesterol synthesis primarily occurs in the cytosol of liver cells.
What is the location of phospholipid synthesis?
Cytoplasmic face of membranes of the endoplasmic reticulum
What is the location of ketone body synthesis?
mitochondria of the liver
How does the body make NADPH for use in fatty acid synthesis?
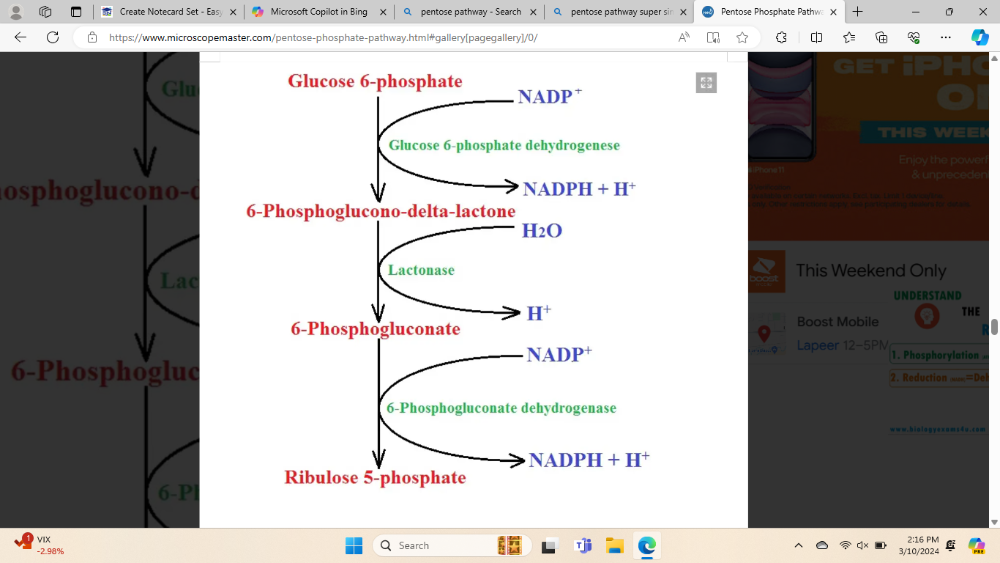
Through the pentose phosphate pathway and by malic enzyme
(it says that I don't really need to know the enzymes and such but I think I should know the names it would make it easier to explain)
Detail the synthesis of fatty acids, including acetyl-CoA carboxylase
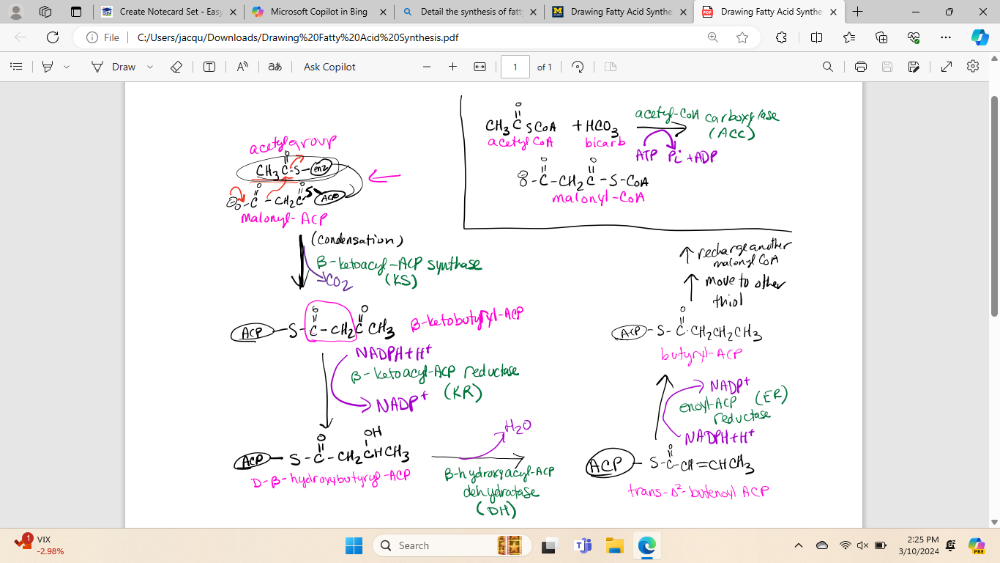
(note: I will need to know all the reactions, all enzyme names, all cofactors, all substates and products)
What is the process of charging ACP prior to fatty acid synthesis
1) acetyl-CoA is converted into malonyl-CoA by the enzyme acetyl-CoA carboxylase (ACC)
2) ACP bind to both acetyl-CoA and malonyl-CoA
3) The phosphopantetheine group of ACP interacts with the substrates, forming acetyl-ACP and malonyl-ACP
4) These loaded ACP molecules serve as carriers for the acetyl and malonyl groups during the elongation process
5) Then these go to fatty acid synthesis
What is the first step of the process of charging ACP prior to fatty acid synthesis
1) acetyl-CoA is converted into malonyl-CoA by the enzyme acetyl-CoA carboxylase (ACC)
What is the second step of the process of charging ACP prior to fatty acid synthesis
2) ACP bind to both acetyl-CoA and malonyl-CoA
What is the third step of the process of charging ACP prior to fatty acid synthesis
3) The phosphopantetheine group of ACP interacts with the substrates, forming acetyl-ACP and malonyl-ACP
What is the fourth step of the process of charging ACP prior to fatty acid synthesis
4) These loaded ACP molecules serve as carriers for the acetyl and malonyl groups during the elongation process
What is the fifth step of the process of charging ACP prior to fatty acid synthesis
5) Then these go to fatty acid synthesis
What are the general details and physiological relevance of the regulation of Acetyl-CoA carboxylase?
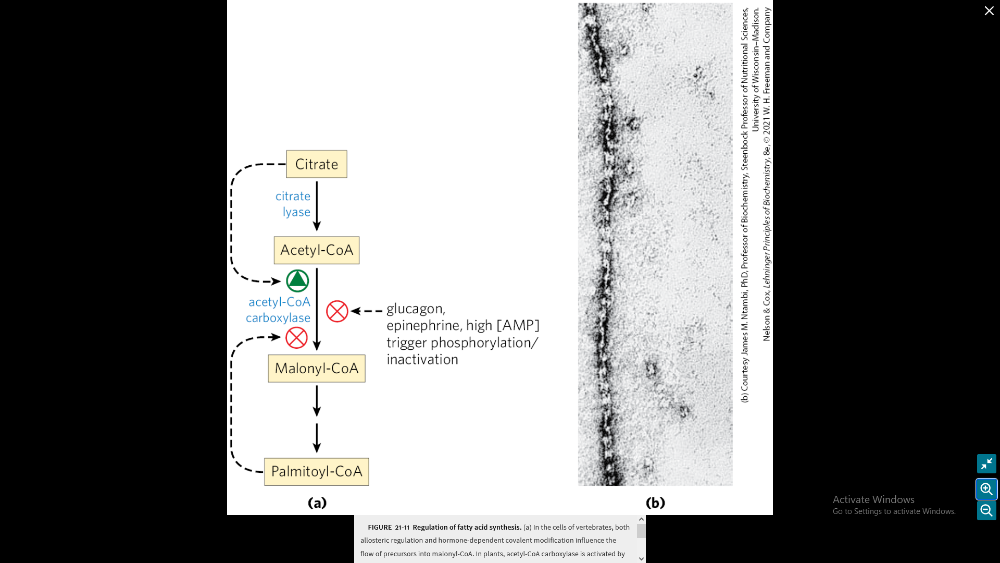
ACC controls pools of malonyls-CoA:
1) inhibition of beta oxidation
2) ACC activates lipid biosynthesis
3) ACC influences lipid storage and mobilization
in regulation of Acetyl-CoA carboxylase what inhibits the making of Malonyl-CoA?
glucagon, epinephrin, high AMP trigger phosphorylation/inactivation, Palmitoyl-CoA
in regulation of Acetyl-CoA carboxylase what activated the creation of acetyl-CoA?
citrate
What are the general details of human fatty acid elongation and desaturation (Know what we can't do)
- Palmitate is the precursor.
- Desaturation involves introduction of double bonds into existing fatty acids
- Human cells can only desaturate fatty acids before the 10th carbon.
- Fatty acid with double bonds near the ends is essential because we can't synthesize them.
- This occurs in the smooth endoplasmic reticulum.
- Human cells lack delta-12 and delta desaturates. We can thus only consume essential omega-3 and omega-6 fatty acids
Detail the formation of eicosanoids from arachidonate, including differentiating between the role of the products of the COX-1 vs. COX-2 reactions.
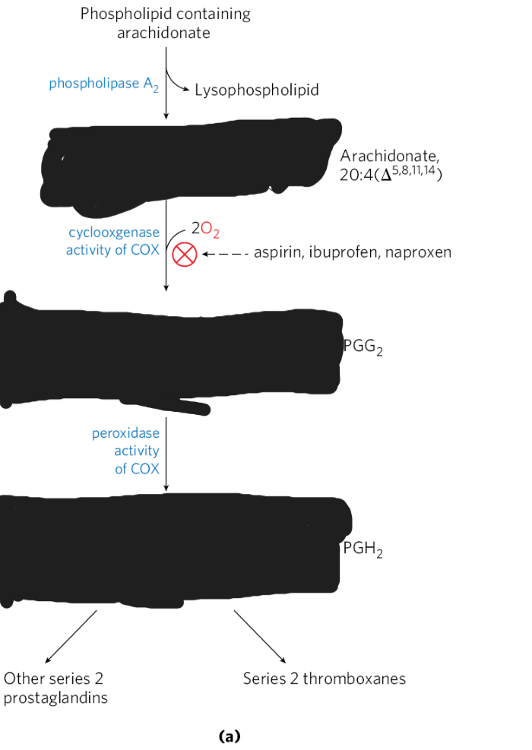
eicosanoids = signaling molecules derived from PUFAs (including arachidonic)
COX = enzymes responsible for converting AA into eicosanoids
1) Cox-1 = regulates the production of prostaglandins, which play a role in platelet aggregation and mucosa acid production
2) Cox-2 = is present in kidneys, brain, and bones. It contributes to inflammation.
(Don't worry about structures)
How does insulin increase synthesis of triglycerides?
- By activating lipogenic enzymes
- stimulates ACC leading to conversion of acetyl-CoA into malonyl-CoA (building block of fatty acid synthesis)
- Insulin enhances uptake of glucose in fat tissue which combines to male triglycerides.
Describe the process of synthesizing phospholipids and triglycerides
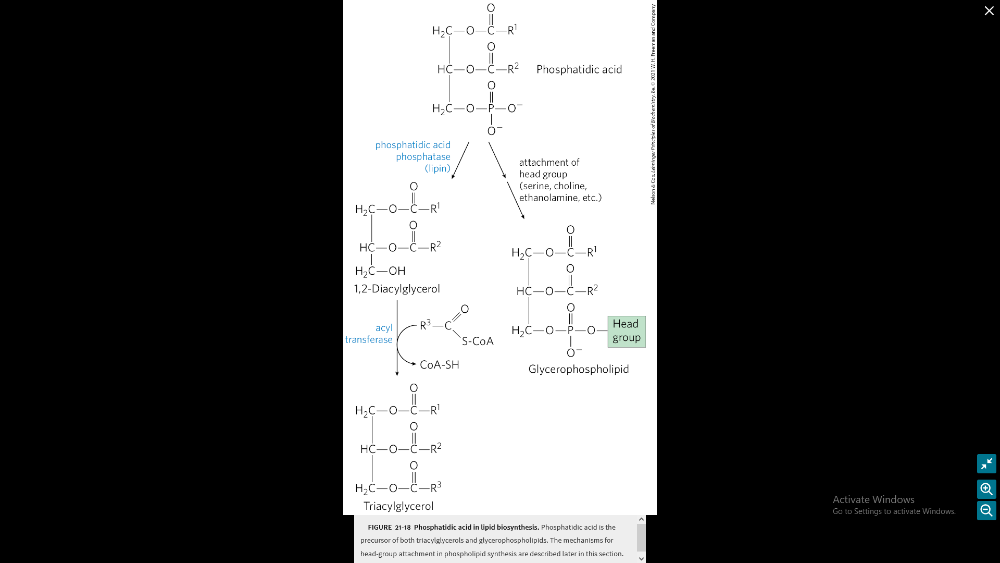
2 precursors = fatty acyl-CoA and L-glycerol 3-phosohate phosphatic acid
Phospholipid Synthesis:
- Starts with adding a fatty acid to glycerol 3-phosphate.
- This creates lysophosphatidic acid.
- All cells make phosphatidylcholine (PC) via the CDP-choline pathway.
- Choline entering the cell is quickly changed to phosphocholine.
Triglyceride Synthesis:
- Made by all cells, but mainly in the intestines and liver.
- Uses NADPH for the reactions.
- NADPH comes from the Pentose Phosphate Pathway and the malic enzyme.
- Triglycerides are made from fatty acyl-CoAs and glycerol-3-phosphate.
glycerolneogenesis: how does it occur?
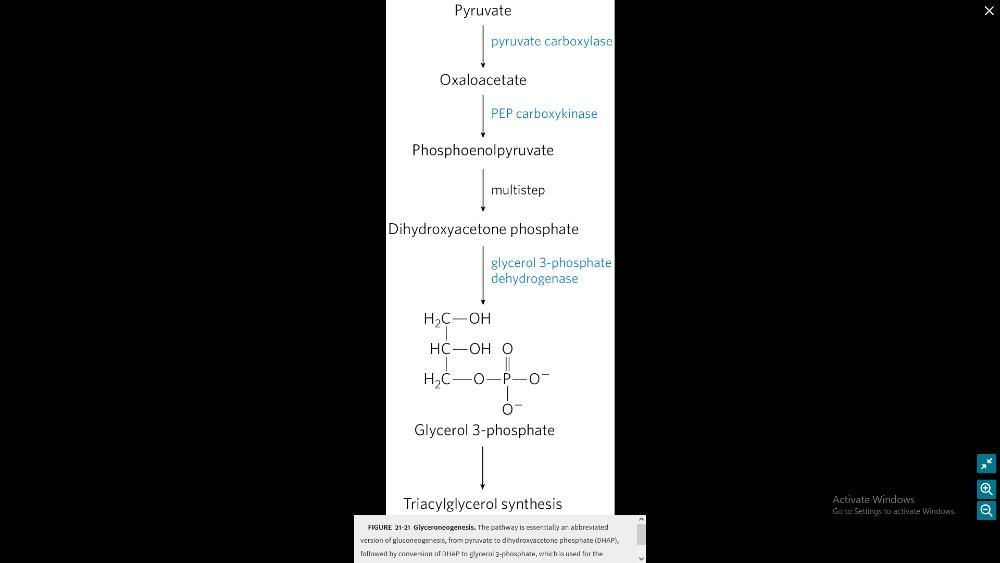
- When glucose is scarce: This process is used when the concentrations of glucose in the cell’s cytoplasm are low1.
- Precursors: The main precursors of glyceroneogenesis are pyruvate, lactate, glutamine, and alanine1.
- Key enzyme: The main regulator enzyme for this pathway is an enzyme called phosphoenolpyruvate carboxykinase (PEPC-K), which catalyzes the conversion of oxaloacetate to phosphoenolpyruvate1.
- Where it happens: Glyceroneogenesis is observed mainly in adipose tissue, and in the liver1.
- Why it’s important: Intense suppression of glyceroneogenesis may lead to metabolic disorders such as type 2 diabetes1.
What is the process of glycerolneogenesis?

- Pyruvate to Oxaloacetate: Pyruvate is converted into oxaloacetate by the enzyme pyruvate carboxylase.
- Oxaloacetate to Phosphoenolpyruvic Acid (PEP): Oxaloacetate is then converted into PEP by the enzyme PEP carboxykinase.
- PEP to Dihydroxyacetone Phosphate: PEP is converted into dihydroxyacetone phosphate through a series of reactions in the glycolysis pathway.
- Dihydroxyacetone Phosphate to Glycerol 3-Phosphate: Dihydroxyacetone phosphate is converted into glycerol 3-phosphate by the enzyme triose phosphate isomerase.
- Glycerol 3-Phosphate to Triglyceride Synthesis: Glycerol 3-phosphate is used as a backbone in the synthesis of triglycerides.
why do we need it glycerolneogenesis?

- Regulates Fatty Acid Release: During periods of fasting, glyceroneogenesis modulates the release of fatty acids. When fuel is not available from the diet, lipolysis releases glycerol and fatty acids from triglyceride stores in fat cells1.
- Triglyceride/Fatty Acid Cycle: Glyceroneogenesis is part of the triglyceride/fatty acid cycle, which includes local intracellular cycling within the adipose tissue and extracellular or systemic recycling. This cycle accounts for a considerable quantity of fatty acid recycling2.
- Prevents Metabolic Disorders: Intense suppression of glyceroneogenesis may lead to metabolic disorders such as type 2 diabetes23.
- Maintains Energy Balance: As well as synthesizing lipids for use in other metabolic processes, glyceroneogenesis regulates lipid levels in the cytosol
how is glycerolneogenesis altered by corticosteroids?

- Induction of PEPC-K in the Liver: Corticosteroids induce the transcription of phosphoenolpyruvate carboxykinase (PEPC-K), the main regulator enzyme for glyceroneogenesis, in the liver1.
(meaning it would downregulate gluconeogenesis)
- Decrease of PEPC-K in Adipose Tissues: At the same time, corticosteroids decrease the transcription of PEPC-K in adipose tissues1.
This dual action of corticosteroids helps to regulate the balance of lipid metabolism between the liver and adipose tissues.
What are the full details of stage 1 of cholesterol synthesis (will need to know reactions, reactants, products, enzyme names) and then a more general description of the other three stages
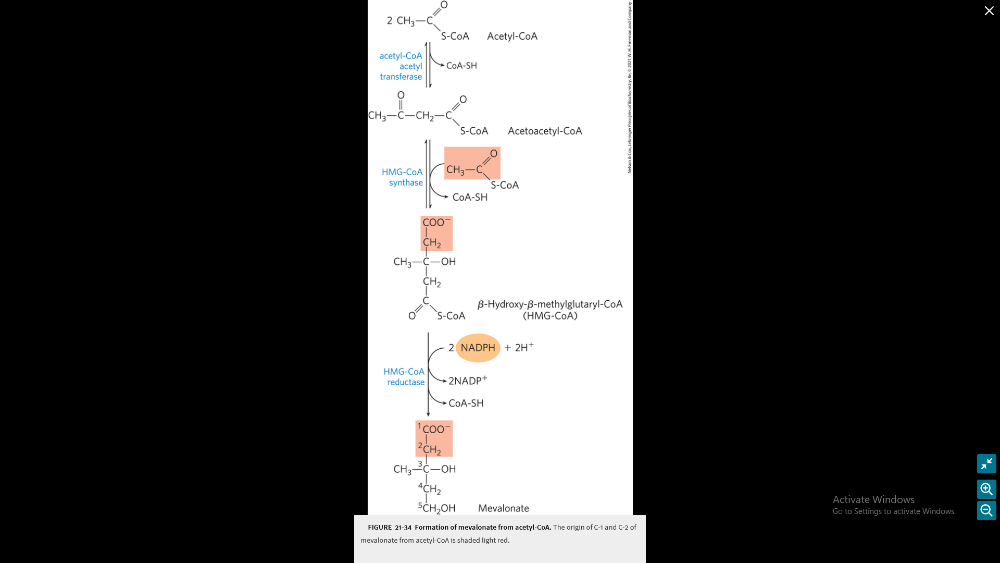
Stage 1: formation of mevalonate from acetyl-CoA
General overview:
- Acetyl-CoA to Acetoacetyl-CoA: Two molecules of acetyl-CoA combine to form acetoacetyl-CoA1.
- Acetoacetyl-CoA to HMG-CoA: Acetoacetyl-CoA is converted into 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) by the enzyme HMG-CoA synthase1.
- HMG-CoA to Mevalonate: HMG-CoA is reduced to mevalonate by the enzyme HMG-CoA reductase. This is the rate-limiting step in cholesterol synthesis23.
- Mevalonate to Isopentenylpyrophosphate (IPP): Mevalonate is converted into IPP2.
- IPP to Squalene: IPP units are joined together to form squalene2.
- Squalene to Cholesterol: Squalene undergoes a series of reactions to form cholesterol2.
Illustrate the composition of a lipoprotein particle
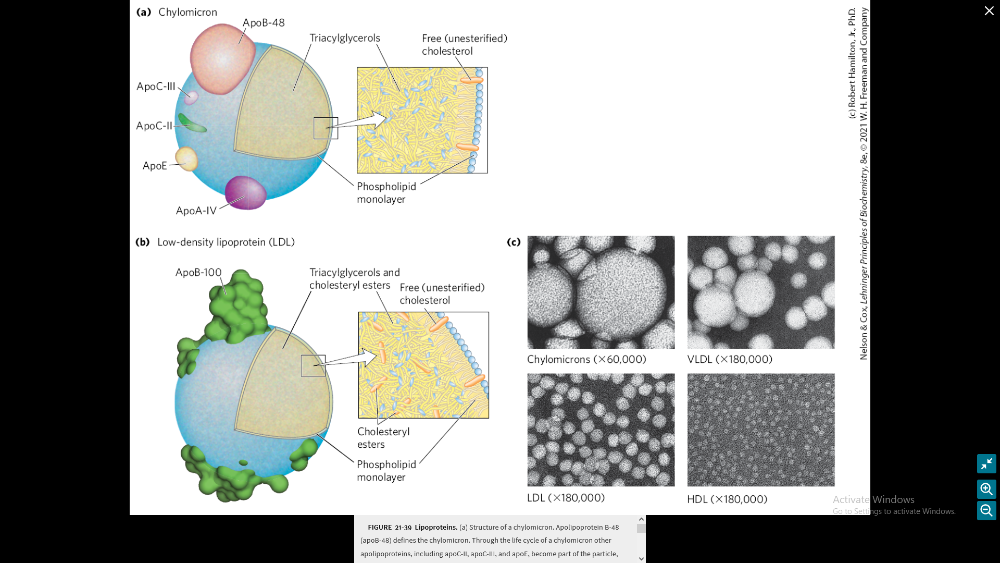
Discuss the lipoprotein trafficking roles (FIGURE 21-40)
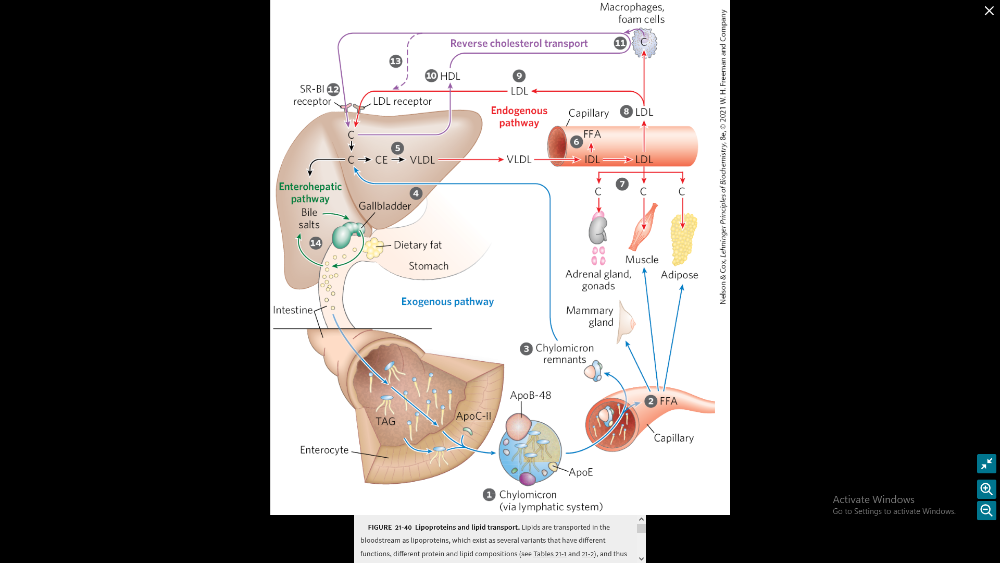
- Lipoproteins: Lipids are transported in the bloodstream as lipoproteins. These exist as several variants with different functions, protein and lipid compositions, and densities.
- Exogenous Pathway:
- Dietary lipids are packaged into chylomicrons.
- Fatty acids from TAG are released by lipoprotein lipase to adipose and muscle tissues during transport through capillaries.
- Chylomicron remnants (which contain largely protein and cholesterol) are taken up by the liver.
- Bile salts produced in the liver aid in dispersing dietary fats and are then reabsorbed in the enterohepatic pathway.
- Endogenous Pathway:
- Lipids synthesized or packaged in the liver are delivered to peripheral tissues by VLDL.
- Extraction of lipid from VLDL gradually converts some of it to LDL.
- LDL delivers cholesterol to extrahepatic tissues or returns to the liver.
- Reverse Cholesterol Transport: Excess cholesterol in extrahepatic tissues is transported back to the liver as HDL.
Describe the uptake of cholesterol and regulation of its synthesis
- Cholesterol Transport: Cholesterol is transported from liver into the bloodstream by lipoproteins such as LDL and VLDL1.
- LDL Receptor-Mediated Endocytosis:
- The LDL receptors on the cell surface bind to LDL particles in the blood1.
- This forms a complex that is internalized into the cell in a process called endocytosis1.
- The complex forms a vesicle inside the cell, which then fuses with a lysosome1.
- The lysosome contains enzymes that break down the LDL, releasing the cholesterol inside the cell1.
-
Regulation of Cholesterol Synthesis:
- The synthesis of cholesterol is regulated by an enzyme called HMG-CoA reductase2.
- When there’s enough cholesterol in the cell, this enzyme is inhibited, which reduces cholesterol synthesis1.
- At the same time, the cell reduces the number of LDL receptors, which decreases cholesterol uptake1.
- If cholesterol levels are low, the cell increases the number of LDL receptors to take up more cholesterol from the bloodstream1.
Describe the molecular basis of familial hypercholesterolemia
- Familial Hypercholesterolemia (FH): It’s an autosomal dominant disorder, meaning you only need one copy of the faulty gene from either parent to develop the condition.
- Gene Mutations: The condition is most commonly caused by mutations in the LDLR gene. Less common are mutations in the APOB and PCSK9 genes.
- LDL Receptor Function: The LDLR gene makes a protein that binds to LDL in the blood and carries it into cells where it’s broken down.
- Effect of Mutations: Mutations in the LDLR gene lead to an abnormal LDL receptor. LDL particles aren’t effectively removed from the bloodstream, leading to high levels of LDL cholesterol.
- Other Genes: Mutations in the APOB gene affect the structure of apolipoprotein B, a protein that binds to the LDL receptor. PCSK9 gene mutations lead to an increase in the number of LDL receptors that are broken down in the cell, reducing the number available to clear LDL cholesterol from the bloodstream.
- Resulting Condition: The end result is high levels of circulating LDL cholesterol, which can lead to the formation of fatty deposits in the arteries, a condition known as atherosclerosis.
Explain why high HDL levels protect against atherosclerosis
- Cholesterol Removal: HDLs remove cholesterol from tissues and transport it back to the liver.
- Inhibition of LDL Oxidation: HDLs prevent the oxidation of LDLs, reducing the formation of harmful foam cells.
- Anti-Inflammatory Properties: HDLs limit the inflammatory processes that underlie atherosclerosis.
- Antithrombotic Properties: HDLs prevent the formation of blood clots, which can lead to heart attacks and strokes.
Name various hormones derived from cholesterol and give their roles
Steroid hormones:
- Testosterone and estrogens = help development of sex characteristic
- Aldosterone = increases blood sodium levels
- cortisol = regulates metabolism, helps with immune response
Thyroid hormones:
- stimulates basal methanolic rate
Vitamin D:
- regulates calcium and phosphate metabolism
What is the process of nitrogen fixation and explain why its needed
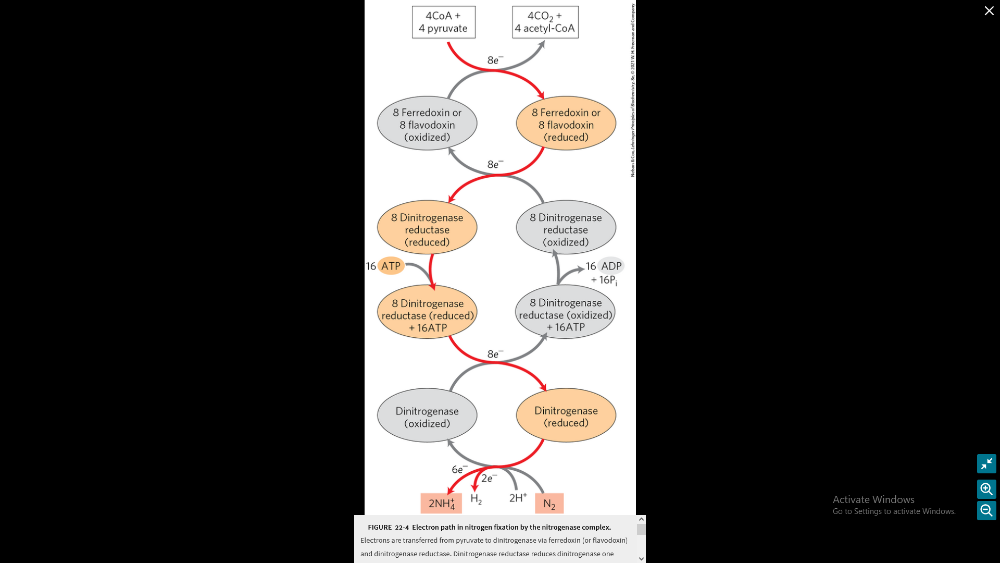
(will need to know the image included)
why its needed:
- nitrogen is a major elemental constituent of living organisms.
- atmosphere is 80% N2, but in Nonuseful form.
- can allow use of renewable energy sources.
- all about converting N2 to NH3, NO2-, or NO-
Draw the reactions showing the incorporation of ammonia into glutamine
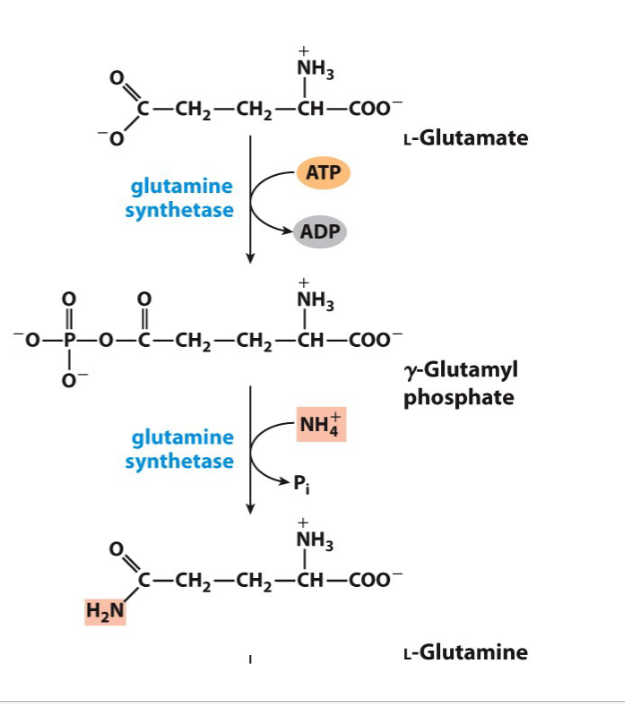
Name the various compounds that inhibit glutamine synthetase
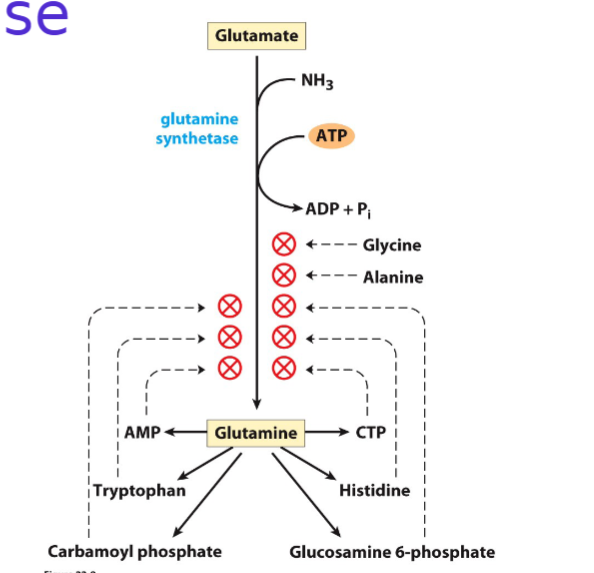
Don't need to draw but I think that is the best option
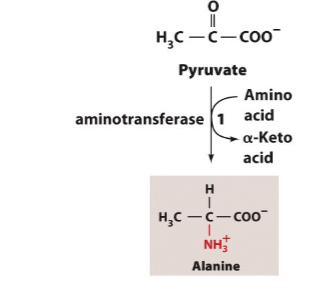
Write the synthesis reactions (with all details) for alanine (from pyruvate)
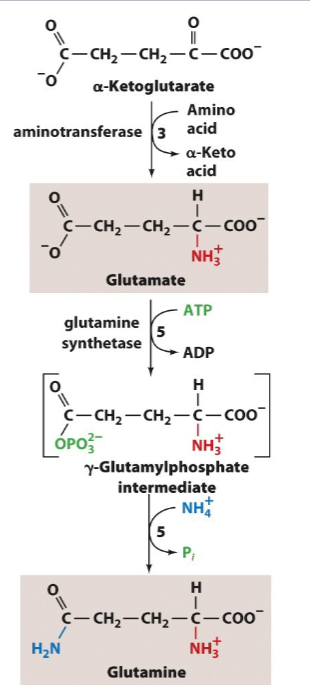
Write the synthesis reactions (with all details) for glutamate and glutamine (from alpha-ketoglutarate),
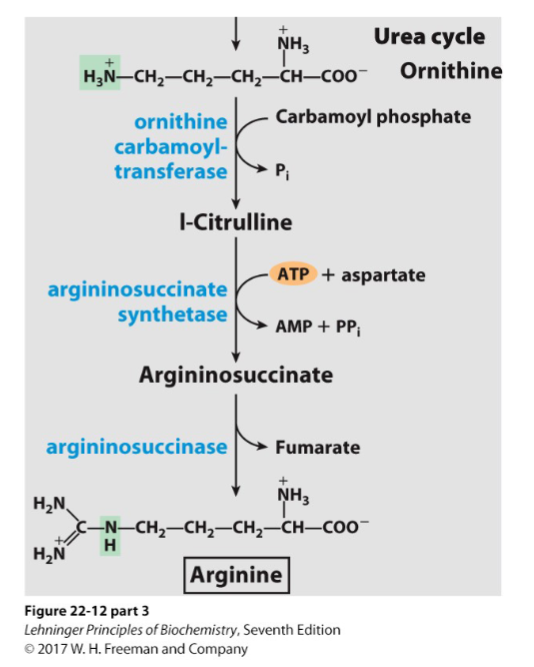
Write the synthesis reactions (with all details) for arginine (from orthenine)
Write the synthesis reactions (with all details) for serine and glycine (from 3-phosphoglkyucerate),
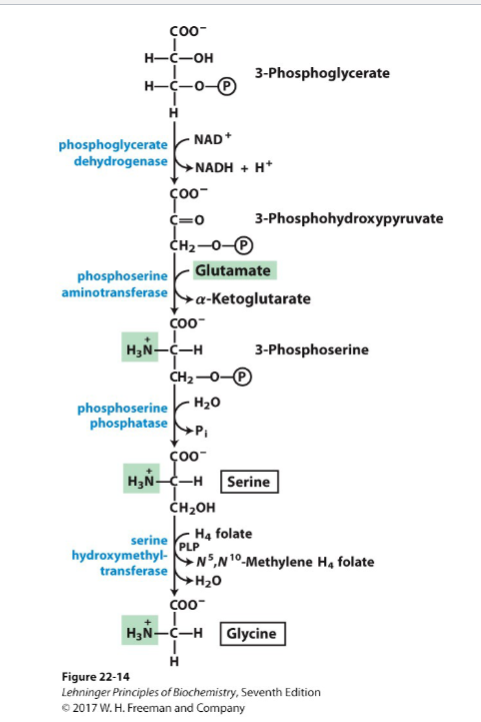
Write the synthesis reactions (with all details) for cysteine (from serine and homocysteine)
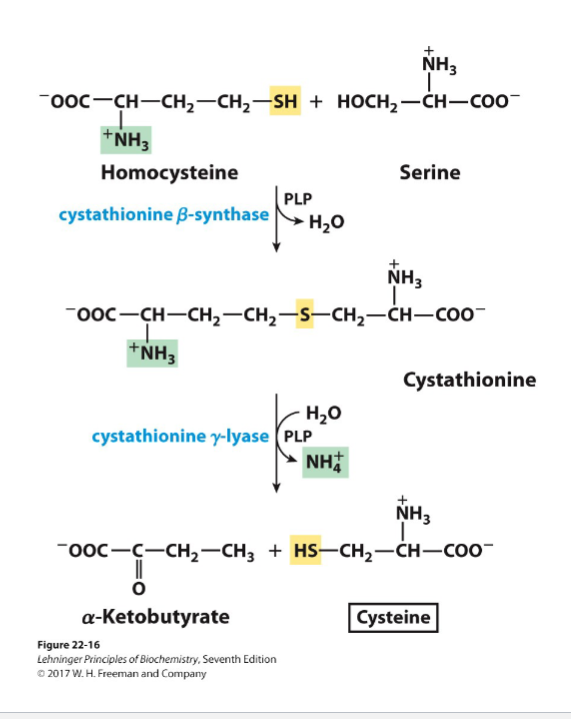
Write the synthesis reactions (with all details) for aspartate and asparagine (from oxaloacetate)
What are several molecules described in book derived from amino acids and give their functions
Porphyrin rings = makes up the heme of hemoglobin, cytochromes, myoglobin (important in Jaundice)
phosphocreatine = important energy buffer om skeletal muscle
glutathione = thought to be a redox buffer
Why might a person develop Jaundice?
impaired liver (liver cancer or hepatitis)
blocked bile secretion (due to gallstones, pancreatic cancer)
insufficient glyceryl bilirubin transferase to process bilirubin (occurs in infants, UGTs)
not breaking down heme
Write the synthesis reaction for serotonin.
Know the function of these neurotransmitters
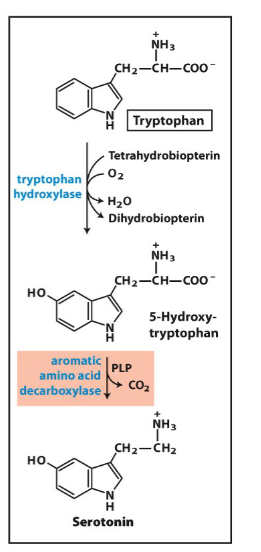
mood and depression involvement
Write the synthesis reaction for dopamine
Know the function of these neurotransmitters
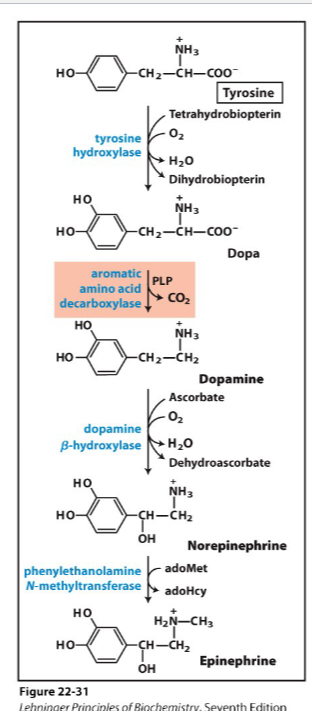
#1 reward center
#2 ability to move the muscles (Parkison's don't make dopamine so they can't move muscles)
Write the synthesis reaction for norepinephrine
Know the function of these neurotransmitters

invovles in thermoregulation signals to the brain
Write the synthesis reaction for epinephrin,
Know the function of these neurotransmitters

used for adrenalin
signals to liver and muscles
fight or flight hormone
Write the synthesis reaction for GABA
Know the function of these neurotransmitters
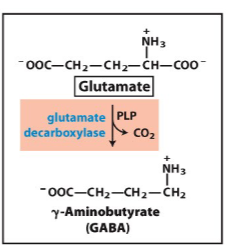
treats seizers
inhibitory transmitter
Write the synthesis reaction for histamine
Know the function of these neurotransmitters
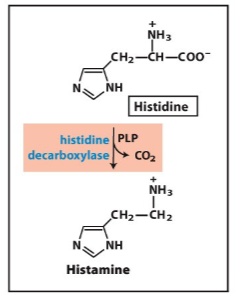
allergic response, acid secretion in stomach (H2 blocker)
Know the primary precursors of all the ring atoms for both purines and pyrimidines
Glucose comes from amino groups.
Glycine is a precursor for purines
asparagine is a precursor for pyrimidines
asparagine is a precursor for
pyrimidines
Glycine is a precursor for
purines
Glucose comes from
amino groups
How is AMP and GMP regulated?
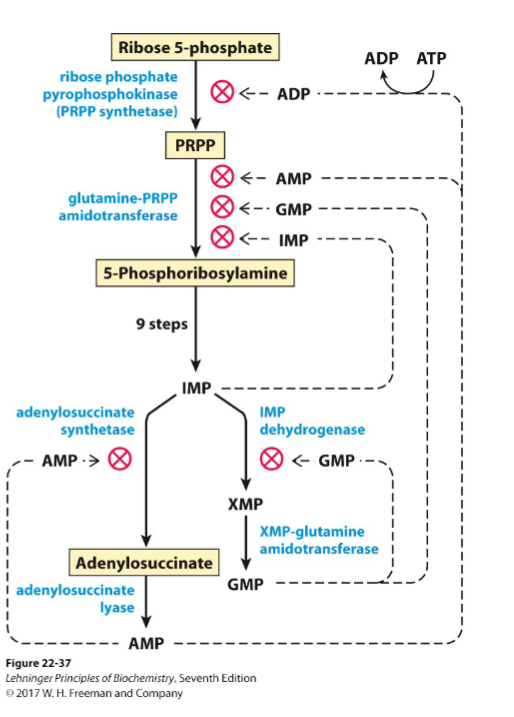
(don't need to know how to draw but I think it is the best way to memorize it)
How are NDPs and NTPs formed from NMPs
adenylate kinase - AMP + ATP <---> 2 ADP
guanylate kinase - GMP + ATP <----> GDP + ADP
nuclease diphosphate kinase = uses various donors of the phosphate group
GDP + ATP <---> GTP + ADP
delta G = 0
Draw the proposed ribonucleotide reductase reaction
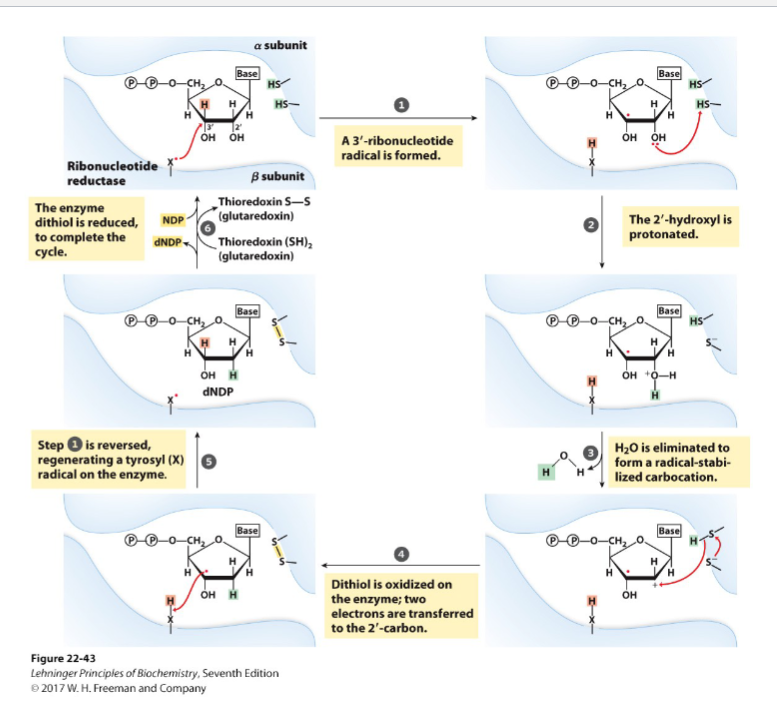
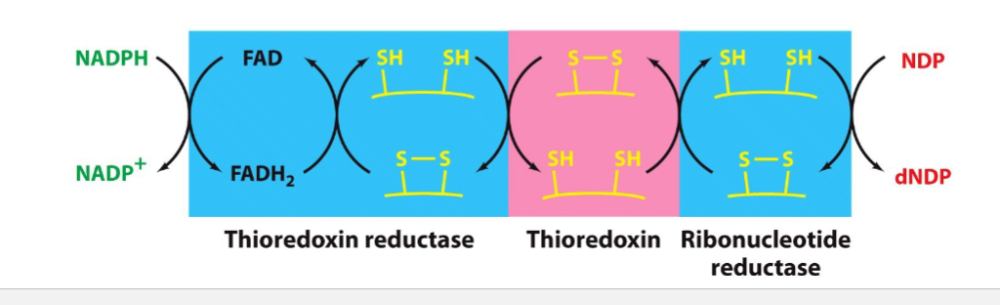
Draw the reoxidation chain for ribonucleotide reductase
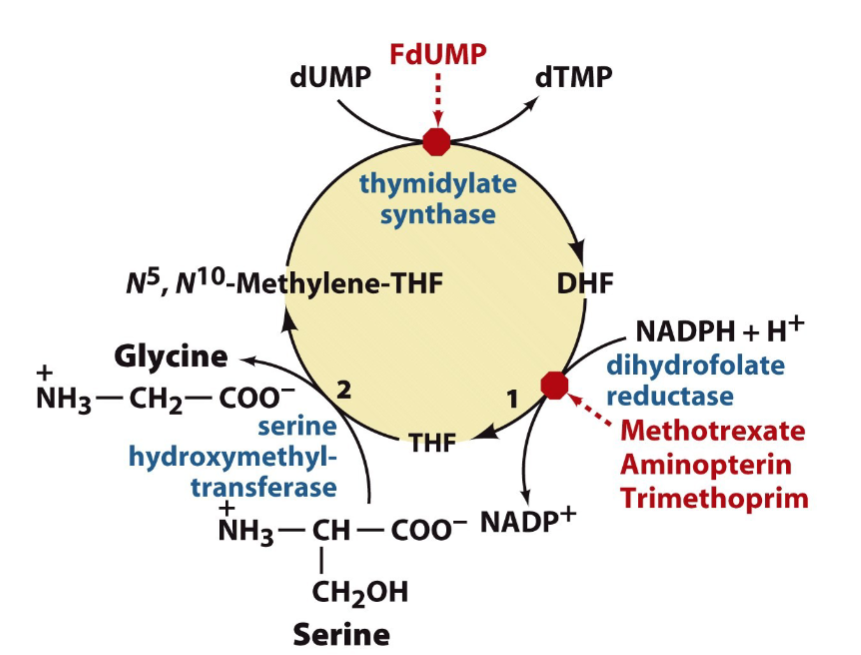
Detail the need for folate to make dTTP
roundabout pathway ...
1) dUTP is made
2) dUTP --> to dUMP by dUTTPase
3) dUMP --> dTMP by thymidylate synthase (target for anti-cancer drugs, no DNA replication = can't divide cells then can't grow)
-adds a methyl group from tetrahydrofolate
Describe the regeneration of tetrahydrofolate, the enzymes invovled and the drugs that inhibit those enzymes - and how they work.