Correctly identify the anatomical sites that are preferentially protected by different antibody isotypes.
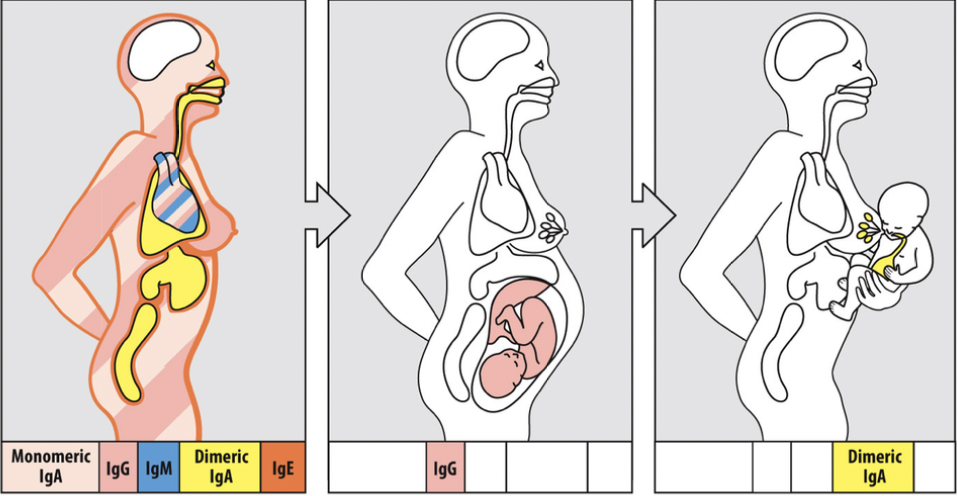
The different classes of antibody tend to protect different tissues of the body (although there is overlap in some cases).
The skin:
- IgE
The heart:
- IgG
- Monomeric IgA
- IgM
The mucosal regions:
- Dimeric IgA
The Fetus:
- IgG
Breastmilk to the baby:
- IgA
The IgG of the fetus is the IgG of the mom.
*the mass cells and the receptor FCER
Describe the process for transporting IgG out of the bloodstream into
a tissue, or across the placenta into the circulation of
the
developing fetus. Understand the location and function of the
neonatal Fc receptor (FcRn).
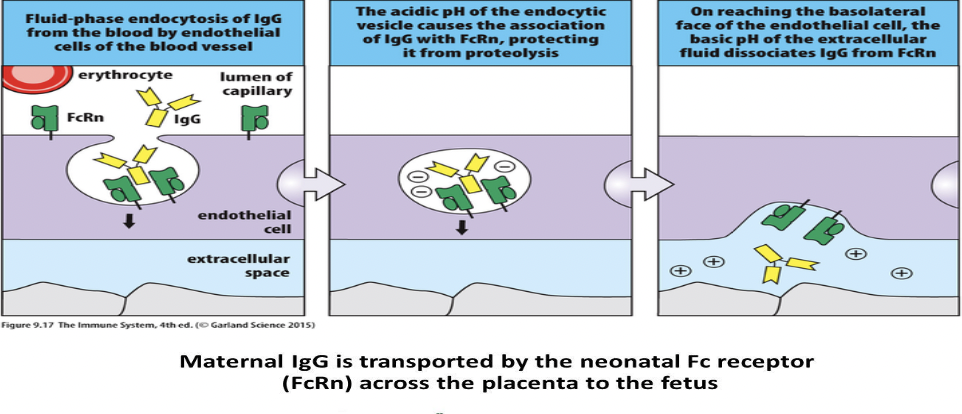
IgG can be transported out of the bloodstream into tissues. Role of the neonatal Fc receptor (FcRn).
- FcRn Location: FcRn is found on the surface of various cells, including endothelial cells in blood vessels, epithelial cells in tissues, and syncytiotrophoblasts in the placenta.
- IgG Binding: FcRn binds to the Fc portion of IgG antibodies in a pH-dependent manner. At acidic pH, such as in endosomes, FcRn binds tightly to IgG. In contrast, at neutral pH, such as in the bloodstream or extracellular spaces, the binding affinity decreases, allowing IgG to be released.
- Transport Across Endothelial or Epithelial Cells: In tissues, FcRn facilitates the transcytosis of IgG antibodies across endothelial or epithelial cells. When IgG binds to FcRn on the luminal side (facing the bloodstream) of these cells, it is internalized via endocytosis. Inside the cell, the acidic pH of endosomes strengthens the interaction between IgG and FcRn, preventing degradation of IgG. The endosome containing IgG then traffics to the opposite side of the cell (abluminal side, facing the tissue interstitium), where the higher pH weakens the IgG-FcRn interaction, leading to the release of IgG into the tissue interstitium.
- Placental Transfer: During pregnancy, FcRn in the syncytiotrophoblasts of the placenta facilitates the transfer of maternal IgG antibodies across the placental barrier to the fetal circulation. Maternal IgG binds to FcRn on the maternal side of the syncytiotrophoblasts, is internalized, and then released on the fetal side, providing passive immunity to the developing fetus.
- Recycling: FcRn also plays a role in the recycling of IgG antibodies. After internalization, instead of being degraded, IgG bound to FcRn is recycled back to the cell surface, where it can be released into the bloodstream or transferred across the placenta again.
In summary, FcRn mediates the transport of IgG antibodies across endothelial or epithelial barriers, such as those in tissues or the placenta, by binding to IgG in a pH-dependent manner, facilitating transcytosis, and preventing degradation, thereby maintaining antibody levels in various compartments and providing passive immunity to developing fetuses.<
Describe the activity of a “neutralizing antibody”.
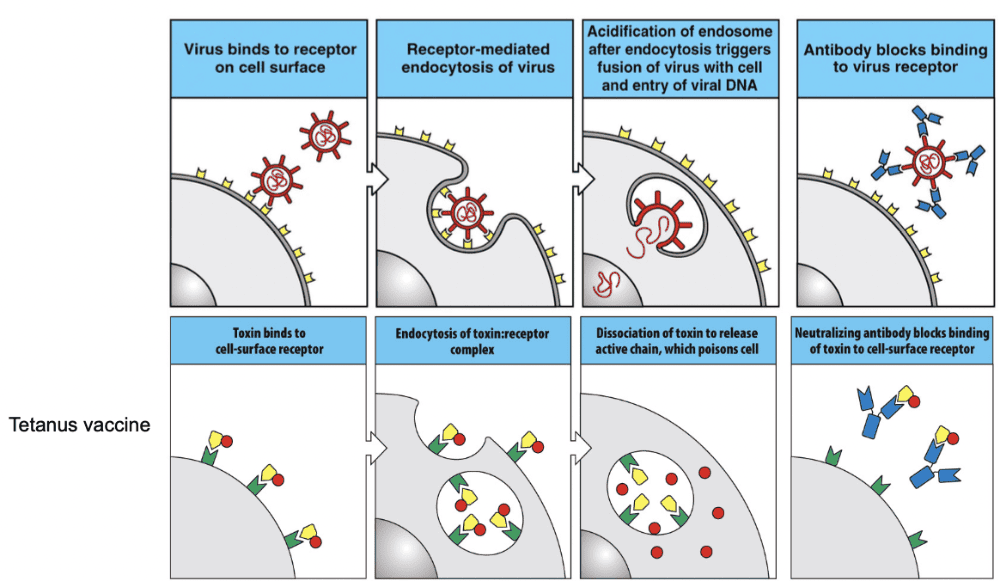
Most are the bonds to viruses
- Binding to Pathogen: Neutralizing antibodies recognize and bind to specific surface molecules or structures on the pathogen, such as viral proteins or toxins. These molecules are often critical for the pathogen's ability to enter host cells or exert its harmful effects.
- Blocking Attachment or Entry: Once bound to the pathogen, neutralizing antibodies can interfere with its ability to attach to host cells or enter them. By blocking key binding sites or masking critical functional domains, neutralizing antibodies prevent the pathogen from engaging with host cell receptors or other necessary molecules.
- Inactivation of Pathogen: Neutralizing antibodies can directly neutralize the infectivity of the pathogen by promoting its aggregation, degradation, or other mechanisms that render it non-functional. This can occur through various processes, such as antibody-mediated complement activation, antibody-dependent cellular cytotoxicity (ADCC), or direct interference with viral replication or toxin activity.
- Enhancement of Immune Response: In addition to their direct antiviral or antibacterial effects, neutralizing antibodies can also contribute to the overall immune response by facilitating the clearance of pathogens by immune cells, such as macrophages or natural killer cells, through mechanisms like opsonization (marking the pathogen for phagocytosis) or by recruiting other immune cells to the site of infection.
Overall, neutralizing antibodies play a crucial role in the immune defense against pathogens by specifically targeting and neutralizing their infectivity, thus helping to prevent or limit the spread of infection and promote the clearance of the pathogen from the body. They are a key component of both natural immunity and vaccine-induced immunity against infectious diseases.
Explain the role of IgG and IgM in activation of the classic
complement cascade. How does the structure of pentameric
IgM
facilitate activation of C1?
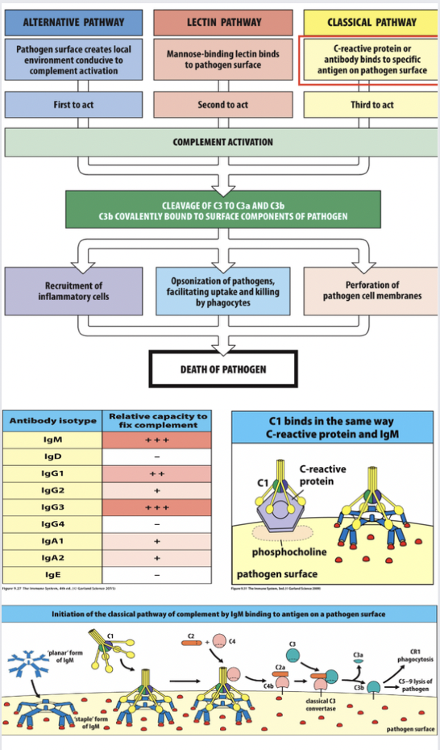
Antibody-mediated activation of the classical complement cascade.
- IgG Activation of Complement Cascade: IgG antibodies can activate the classical complement pathway by binding to antigens on the surface of pathogens or other targets. When IgG antibodies bind to their specific antigen, they undergo a conformational change that exposes their Fc region. This exposed Fc region can then bind to the C1 complex, which consists of C1q, C1r, and C1s. This binding initiates a cascade of proteolytic activations that ultimately lead to the formation of the membrane attack complex (MAC) and lysis of the target cell.
- IgM Activation of Complement Cascade: IgM antibodies are particularly efficient at activating the complement cascade due to their pentameric structure. IgM molecules are composed of five antibody units held together by a J chain. Each IgM molecule has ten antigen-binding sites, which allows for multivalent binding to antigens. This multivalency increases the avidity of IgM for antigens and enhances its ability to cross-link antigens on the surface of pathogens. When multiple IgM antibodies bind to antigens, they can efficiently recruit and activate the C1 complex, leading to rapid complement activation.
Regarding the structure of pentameric IgM facilitating activation of C1: The pentameric structure of IgM enhances complement activation through two main mechanisms:
- Multivalency: Each IgM molecule has ten antigen-binding sites, allowing it to bind to multiple antigens simultaneously. This multivalent binding increases the likelihood of cross-linking antigens, which is necessary for efficient activation of the C1 complex.
- Spatial Arrangement: The arrangement of IgM subunits in a pentamer facilitates the formation of a stable complex with the C1 complex. The close proximity of IgM molecules in the pentameric structure ensures efficient binding to multiple C1 complexes, promoting complement activation.
Overall, the pentameric structure of IgM enhances complement activation by facilitating multivalent binding to antigens and promoting efficient interaction with the C1 complex.
Explain how some isotypes of antibody function as opsonins. Explain the role of Fc receptors and innate cell types in this process.
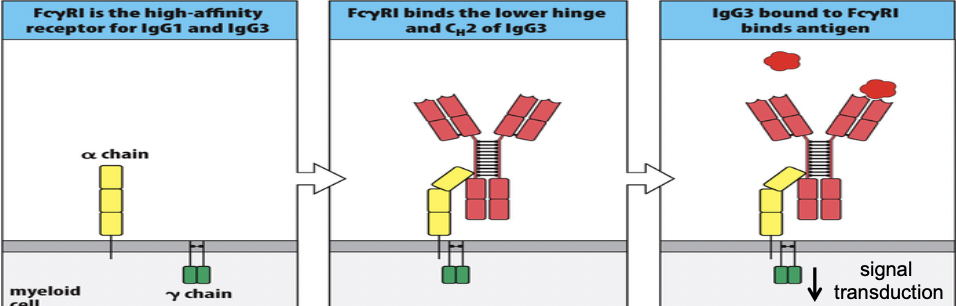
Some isotypes of antibody can function as opsonins. This requires interactions between Ab and Fc-receptors expressed on phagocytic cells.
FCˠ= FC gamma
Enhanced phagocytosis of Ab and complement (C3b) coated pathogens.
- Opsonization: Antibodies, particularly IgG and IgM, bind specifically to antigens on the surface of pathogens or other targets. This binding process is facilitated by the variable regions of the antibody molecules, which recognize and bind to specific epitopes on the antigen. Once bound, the antibodies act as opsonins, coating the surface of the target and tagging it for recognition by phagocytic cells.
- Recognition by Fc Receptors: Phagocytic cells express Fc receptors on their surface, which specifically bind to the Fc region of antibodies. The Fc region is the constant region of the antibody molecule, located opposite to the antigen-binding sites. When antibodies bound to antigens on the surface of pathogens engage with Fc receptors on phagocytic cells, it triggers a series of signaling events within the phagocyte.
- Phagocytosis: Engagement of Fc receptors by antibody-opsonized targets triggers phagocytic cells to engulf the antibody-coated target through a process called phagocytosis. This engulfment leads to the formation of a phagosome, an intracellular vesicle containing the ingested target.
- Degradation and Elimination: Once the target is internalized within the phagosome, it becomes subjected to the antimicrobial mechanisms of the phagocyte, which may involve the production of reactive oxygen species, acidification of the phagosome, and enzymatic degradation. Eventually, the degraded remnants of the target are eliminated from the phagocyte through exocytosis or processed for antigen presentation to activate adaptive immune responses.
The role of Fc receptors and innate cell types in this process is crucial:
- Fc Receptors: Fc receptors are membrane-bound receptors expressed on the surface of various immune cells, including macrophages, neutrophils, dendritic cells, and natural killer cells. These receptors specifically bind to the Fc region of antibodies, thereby mediating the interaction between opsonized targets and phagocytic cells. Different Fc receptor subtypes have dist
Describe the fundamental process of ADCC (antibody-mediated cellular
cytotoxicity). What types of immune cells can participate
in
ADCC, what roles are played by Ab, Fc receptors, perforin/granzyme B?
How is a cell actually killed in the ADCC process
(apoptosis most often)?
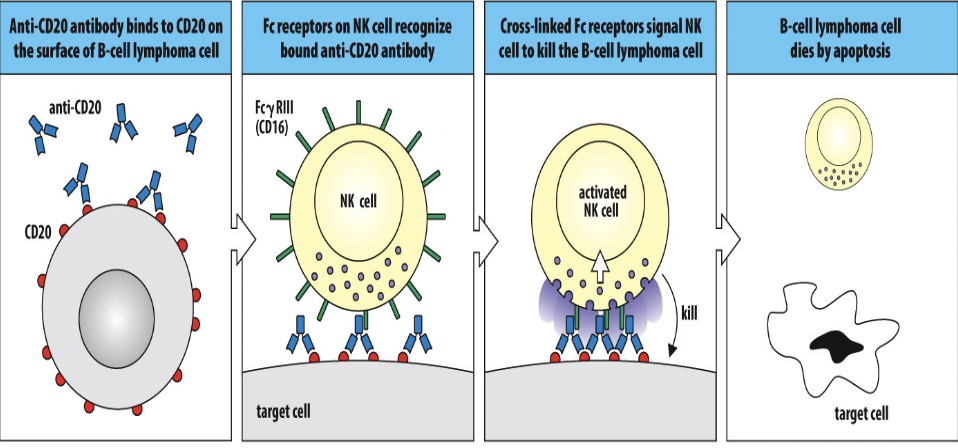
- Opsonization: The process begins with the binding of antibodies, primarily IgG subclasses (e.g., IgG1, IgG3), to specific antigens present on the surface of target cells, such as infected or cancerous cells. This binding is mediated by the antigen-binding sites of the antibodies, which recognize and bind to epitopes on the target cells.
- Recognition by Fc Receptors: Immune effector cells, such as natural killer (NK) cells, macrophages, and neutrophils, express Fc receptors (FcγRIII or CD16) on their surface. These receptors specifically bind to the Fc region of antibodies that are bound to the surface of target cells. This interaction between Fc receptors on effector cells and antibody-bound target cells initiates the ADCC process.
- Activation of Effector Cells: Upon binding to antibody-coated target cells, the effector cells become activated. This activation triggers the release of cytotoxic molecules and initiates the killing process. NK cells are particularly important mediators of ADCC due to their ability to induce apoptosis in target cells.
- Release of Cytotoxic Molecules: Activated effector cells release cytotoxic molecules, such as perforin and granzyme B, towards the antibody-bound target cells. Perforin forms pores in the cell membrane of the target cell, facilitating the entry of granzyme B and other cytotoxic molecules into the target cell's cytoplasm.
- Induction of Apoptosis: Once inside the target cell, granzyme B activates caspases, a group of protease enzymes, which initiate a cascade of events leading to apoptosis, or programmed cell death, of the target cell. Apoptosis is the preferred mechanism of cell death in ADCC because it allows for the efficient removal of the target cell without causing inflammation or damage to surrounding tissues.
In summary, ADCC is a process in which immune effector cells, such as NK cells, recognize and destroy target cells coated with antibodies through the engagement of Fc receptors, release of cytotoxic molecules like perforin and granzyme B, and induction of apoptosis in the target cells. This mechanism plays a critical role in immune surveillance and defense against infections and cancer.
Describe the unique function of IgE antibodies in combating worm
infections. What is the role of the Fc epsilon receptor in
this
process? What types of immune cells participate in this
process (the eosinophil mostly)?
IgE antibodies play a unique role in combating worm infections through a process called helminth immunity. When the body encounters parasitic worm infections, such as helminths, it initiates an immune response characterized by the production of IgE antibodies.
The unique function of IgE antibodies in this context is their ability to bind to specific receptors called Fc epsilon receptors (FcεR) found on the surface of immune cells, particularly mast cells and basophils. When IgE antibodies bind to FcεR, it sensitizes these cells to respond rapidly upon subsequent exposure to the same worm antigens.
During a worm infection, the worm antigens trigger the cross-linking of IgE antibodies bound to FcεR on mast cells and basophils. This cross-linking leads to the release of potent mediators such as histamine, leukotrienes, and cytokines from these cells. These mediators play crucial roles in orchestrating an immune response against the worms.
Eosinophils are another type of immune cell that plays a significant role in combating worm infections, particularly through their interaction with IgE antibodies. Eosinophils express receptors for IgE antibodies, allowing them to bind to IgE-opsonized parasites. Upon binding, eosinophils release toxic granule contents, including major basic protein and eosinophil peroxidase, which are highly effective at killing parasites.
In summary, IgE antibodies facilitate the immune response against worm infections by sensitizing mast cells, basophils, and eosinophils through binding to FcεR. This sensitization primes these cells to respond rapidly and efficiently upon subsequent exposure to worm antigens, leading to the release of mediators and toxic granule contents that help combat the infection.
Describe the location of microfold cells (M cells) and describe the
role that these specialized cells play in antigen delivery
to
mucosa-associated secondary lymphoid tissues (e.g. Peyer’s
patches, tonsils). This is the transcytosis process
Microfold cells (M cells) are specialized epithelial cells found in the epithelium that covers mucosa-associated lymphoid tissues, such as Peyer's patches in the intestines and the tonsils in the upper respiratory tract.
The location of M cells within these tissues is strategically positioned over the lymphoid follicles, which are regions rich in immune cells like B and T lymphocytes. M cells are characterized by their unique morphology, featuring a distinct microfold or "M" shape on their apical surface, which gives them their name.
The primary role of M cells is to transport antigens and pathogens from the lumen of the mucosa across the epithelial barrier to the underlying lymphoid tissue, where they can initiate an immune response. This process, known as transcytosis, involves several steps:
- Antigen Sampling: M cells actively sample antigens, including bacteria, viruses, and other foreign particles, from the mucosal lumen. These antigens may be present in the food or liquid passing through the gut or inhaled through the respiratory tract.
- Uptake and Transport: Once antigens are captured, M cells internalize them through endocytosis or phagocytosis. The antigens are then transported across the M cell and released into the subepithelial space on the basolateral side.
- Presentation to Immune Cells: In the subepithelial space, antigens encounter antigen-presenting cells (APCs), such as dendritic cells, which capture and process the antigens. The APCs then present antigenic peptides derived from the antigens to T cells, initiating an adaptive immune response.
- Activation of Immune Response: The presentation of antigens to T cells leads to the activation and differentiation of T cells into effector cells, such as helper T cells or cytotoxic T cells. These T cells, in turn, coordinate immune responses by activating B cells, inducing the production of antibodies, and recruiting other immune cells to the site of infection.
- Induction of Immune Tolerance: In addition to initiating immune responses against pathogens, M cells also play a role in inducing immune tolerance to harmless antigens, such as food proteins or commensal bacteria. This process helps prevent inappropriate immune reactions and maintains the balance of the immune system.
Overall, M cells are essential for surveilling the mucosal environment and delivering antigens to mucosa-associated secondary lymphoid tissues, where they initiate immune responses and help maintain mucosal homeostasis.
Describe the general steps that are involved in the activation of
naïve B and T lymphocytes in mucosa-associated lymphoid
tissues
(essentially the same as what they are in the lymph node
as discussed in earlier lectures).
- Antigen Encounter: The process begins with the encounter of naive B and T lymphocytes with antigens presented within the mucosa-associated lymphoid tissues. Antigens may be derived from pathogens, commensal microorganisms, or food particles.
- Antigen Uptake and Presentation: Antigen-presenting cells (APCs), such as dendritic cells, macrophages, or specialized epithelial cells like M cells, capture antigens from the mucosal lumen. These APCs process the antigens into smaller peptide fragments and present them on their surface bound to major histocompatibility complex (MHC) molecules.
- Migration to T Cell Zone: Naive T lymphocytes migrate from the bloodstream into the mucosa-associated lymphoid tissues and move toward the T cell zone, guided by chemokines secreted by APCs and stromal cells.
- T Cell Activation: Within the T cell zone, naive T lymphocytes encounter APCs presenting antigen-MHC complexes. T cell receptor (TCR) recognition of specific peptide-MHC complexes, along with co-stimulatory signals provided by APCs (e.g., CD80/CD86 binding to CD28 on T cells), triggers T cell activation.
- Clonal Expansion: Activated T cells undergo clonal expansion, resulting in the proliferation of antigen-specific T cell clones. This proliferation amplifies the number of effector T cells capable of responding to the antigen.
- Differentiation into Effector T Cells: The activated T cells differentiate into effector T cells with specialized functions, such as helper T cells (e.g., Th1, Th2, Th17) or cytotoxic T cells (CD8+), depending on the cytokine milieu and the nature of the antigen.
- Migration of Effector T Cells: Effector T cells exit the mucosa-associated lymphoid tissues and migrate to the site of infection or inflammation, where they exert their effector functions to eliminate pathogens or modulate immune responses.
- B Cell Activation: Meanwhile, naive B lymphocytes encounter antigens presented by follicular dendritic cells or antigen-specific T helper cells within the germinal centers of mucosa-associated lymphoid tissues. This interaction, along with co-stimulatory signals, leads to B cell activation.
- Clonal Expansion and Differentiation: Activated B cells undergo clonal expansion and differentiate into plasma cells, which secrete antibodies specific to the encountered antigen, or memory B cells, which persist for long-term immunity.
- Antibody Production and Immune Memory: Plasma cells secrete antibodies into the mucosal environment, where they neutralize pathogens or toxins. Memory B cells provide long-term immune memory, enabling a rapid and robust response upon re-exposure to the same antigen.
Overall, the activation of naive B and T lymphocytes in mucosa-associated lymphoid tissues involves a coordinated interplay between antigen presentation, T cell activation, B cell activation, clonal expansion, differentiation, and effector functions, ultimately leading to the generation of adaptive immune responses tailored to mucosal pathogens and antigens.
Describe the mechanism for transport of sIgA and IgM onto mucosal
surfaces. Describe the roles of the poly-Ig receptor, J-
chain,
transcytosis, and protease enzymes in this process.
- Poly-Ig Receptor (pIgR) Binding: The process begins with the binding of polymeric immunoglobulin receptors (pIgR) to the Fc portion of dimeric IgA or pentameric IgM antibodies. These pIgRs are expressed on the basolateral surface of epithelial cells lining mucosal tissues.
- Internalization of pIgR-Antibody Complex: Upon binding to pIgR, the pIgR-antibody complex undergoes internalization into epithelial cells via receptor-mediated endocytosis.
- Formation of Endosomal Vesicles: Within the epithelial cells, the pIgR-antibody complex is internalized into endosomal vesicles, where the complex undergoes proteolytic cleavage mediated by protease enzymes.
- Cleavage and Release of Secretory Component (SC): Proteolytic cleavage of the pIgR within the endosomal vesicles results in the release of a fragment called the secretory component (SC). The SC remains associated with the bound IgA or IgM antibody, forming a complex known as secretory IgA (sIgA) or secretory IgM (sIgM).
- J-Chain Association: Prior to transcytosis, the J-chain protein associates with the IgA or IgM antibodies bound to SC. The J-chain facilitates the polymerization of IgA or IgM antibodies into dimers (in the case of IgA) or pentamers (in the case of IgM), enhancing their stability and function.
- Transcytosis: The pIgR-SC-IgA/IgM complex is transported across the epithelial cell via a process called transcytosis. Transcytosis involves the movement of vesicles containing the complex from the basolateral surface of the epithelial cell to the apical surface, where they are released into the mucosal lumen.
- Secretion onto Mucosal Surface: Upon reaching the apical surface of the epithelial cell, the vesicles containing the pIgR-SC-IgA/IgM complex fuse with the plasma membrane, releasing the complex into the mucosal lumen. Here, sIgA and sIgM antibodies are present in high concentrations, providing localized protection against pathogens and antigens.
In summary, the transport of sIgA and sIgM onto mucosal surfaces involves the binding of pIgR to IgA or IgM antibodies, internalization, proteolytic cleavage to release SC, association with the J-chain, transcytosis across epithelial cells, and secretion into the mucosal lumen. This process ensures the delivery of antibody-mediated immune protection to mucosal surfaces, where pathogens often enter the body.
Be aware of the fact that some IgG is also transported onto mucosal surfaces, but less than sIgA and IgM.
Certainly! While secretory immunoglobulin A (sIgA) and immunoglobulin M (IgM) are the primary immunoglobulins transported onto mucosal surfaces, some immunoglobulin G (IgG) is also transported, although to a lesser extent. IgG transport onto mucosal surfaces typically occurs via a process known as transudation, where small amounts of IgG from the bloodstream pass through the epithelial barrier into mucosal secretions.
The mechanism of IgG transudation involves the diffusion of IgG molecules across the epithelial barrier, facilitated by their smaller size compared to polymeric IgA and IgM antibodies. This passive diffusion allows for the presence of IgG in mucosal secretions, albeit at lower concentrations than sIgA and IgM.
Although IgG is less abundant in mucosal secretions, it still plays important roles in mucosal immunity. IgG antibodies present in mucosal secretions can neutralize pathogens, toxins, and allergens, providing additional protection against mucosal infections and allergenic responses.
In summary, while sIgA and IgM are the predominant immunoglobulins transported onto mucosal surfaces, some IgG is also present, albeit in lower concentrations. This underscores the diversity and complexity of mucosal immune responses and highlights the complementary roles of different antibody isotypes in mucosal immunity.
ChatGPT can make mistakes. Conside
Explain the immunological benefits of having antibody localized to
the mucosal epithelium surface. What types of infections
are
these antibodies particularly useful against?
Having antibodies localized to the mucosal epithelium surface provides several immunological benefits, enhancing the body's defense against a variety of pathogens and contributing to mucosal immunity:
- Barrier Protection: Mucosal antibodies, particularly secretory immunoglobulin A (sIgA) and immunoglobulin M (IgM), form a protective barrier on mucosal surfaces, such as the respiratory, gastrointestinal, and genitourinary tracts. This barrier prevents pathogens from adhering to and invading the mucosal epithelium, thereby blocking the initial steps of infection.
- Neutralization of Pathogens: Mucosal antibodies can neutralize pathogens by binding to their surface antigens, preventing them from infecting host cells or tissues. Neutralization can occur through various mechanisms, including blocking pathogen binding to host receptors, inhibiting pathogen motility, or interfering with pathogen replication.
- Immune Exclusion: Mucosal antibodies contribute to the process of immune exclusion, where pathogens are trapped and immobilized within the mucosal mucus layer. This prevents pathogens from penetrating the mucosal barrier and facilitates their clearance by mucociliary clearance mechanisms or by subsequent immune responses.
- Agglutination and Precipitation: Mucosal antibodies can promote the agglutination or precipitation of pathogens, causing them to clump together or form insoluble complexes. This enhances the clearance of pathogens by facilitating their recognition and uptake by phagocytic cells, such as macrophages and neutrophils, or by promoting their clearance through mucociliary clearance mechanisms.
- Enhancement of Immune Responses: Mucosal antibodies can enhance local immune responses by interacting with other components of the mucosal immune system, such as dendritic cells, macrophages, and T cells. This can lead to the activation of innate immune responses, such as the secretion of antimicrobial peptides and cytokines, as well as the induction of adaptive immune responses, including the recruitment and activation of effector T cells and the generation of memory B cells.
Mucosal antibodies are particularly useful against infections caused by pathogens that enter the body through mucosal surfaces, such as:
- Respiratory Infections: Mucosal antibodies help protect against respiratory pathogens, including viruses (e.g., influenza virus, respiratory syncytial virus), bacteria (e.g., Streptococcus pneumoniae, Haemophilus influenzae), and fungi (e.g., Candida albicans).
- Gastrointestinal Infections: Mucosal antibodies play a critical role in preventing gastrointestinal infections caused by bacteria (e.g., Vibrio cholerae, Salmonella spp., Escherichia coli), viruses (e.g., rotavirus, norovirus), and parasites (e.g., Giardia lamblia, Cryptosporidium spp.).
- Genitourinary Infections: Mucosal antibodies help protect against genitourinary tract infections caused by bacteria (e.g., Neisseria gonorrhoeae, Chlamydia trachomatis), viruses (e.g., herpes simplex virus, human papillomavirus), and fungi (e.g., Candida albicans).
In summary, mucosal antibodies provide localized protection against a wide range of pathogens at mucosal surfaces, contributing to mucosal immunity and helping to prevent mucosal infections. Their diverse mechanisms of action make them effective in neutralizing pathogens, enhancing clearance mechanisms, and promoting immune responses against mucosal pathogens.
Class-switching does not change what an antibody binds to (fact). True/False: Class-switching can eliminate a function of an antibody and it can create a new function for the antibody.
True
A woman receives the COVID mRNA vaccine. Two months after receiving the vaccine the woman becomes pregnant and eventually gives birth to a little boy that she names Bart. True/False: On the day that Bart is born, he has plasma cells in his body that are secreting antibodies specific for proteins of the COVID (SARS-CoV2) virus.
False
IgM and IgG can both activate the classic complement cascade (fact). True/False: IgG is better than IgM at activating the classic complement cascade when antibody is present at very low levels.
False
Individuals who lack a functional gene encoding a protein called RANKL produce little to no M cells (fact). True/False: These individuals would not be expected to make a normal antibody response after receiving the COVID-19 vaccine.Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.aTruebFalse
false
Which one of the following situations is not an example of passive immunity.
a.Administering human serum obtained from COVID survivors to treat a COVID infection.
b.Transferring plasma cells from one mouse into a second mouse.
c.Administering an mRNA vaccine.
d.Transfer of maternal IgG across the placenta and into the fetus.
e.Ingestion of sIgA antibodies by a child during breast feeding.
c.Administering an mRNA vaccine.
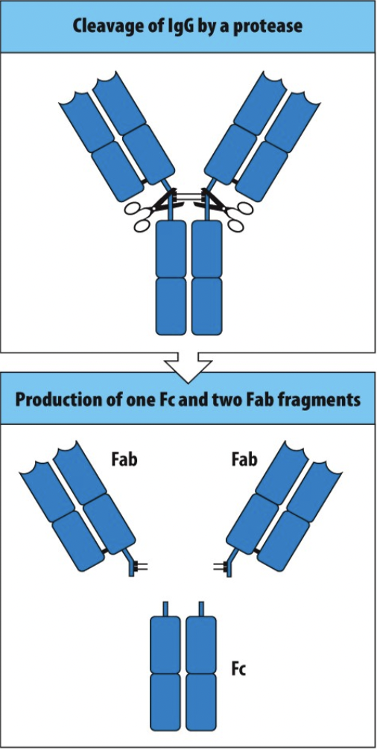
An antibody can be separated (cleaved) into Fab fragments and Fc fragments by treating them with enzymes such as papain (fact). You digest an antibody that binds to a neutralizing epitope on SARS-CoV2 with papain. Which one of the following statements is true
a.The Fc fragment of this antibody retains the ability to neutralize SARS-CoV2 virus.
b.The Fab fragments of this antibody retain the ability to neutralize SARS-CoV2 virus.
c.Both the Fc fragment and the Fab fragments of this antibody retain the ability to neutralize SARS-CoV2 virus.
d.Neither the Fc fragment nor the Fab fragments of this antibody retain the ability to neutralize SARS-CoV2 virus.
b.The Fab fragments of this antibody retain the ability to neutralize SARS-CoV2 virus.

An antibody can be separated (cleaved) into Fab fragments and Fc fragments by treating them with enzymes such as papain (fact). You digest an antibody that binds to a virus protein that is expressed on the surface of a cell infected with virus X and helps to kill those cells by participating in ADCC. Which one of the following statements is true?
a.The Fc fragment of this antibody retains the ability to kill virus infected cells by participating in ADCC.
b.The Fab fragments of this antibody retain the ability to kill virus infected cells by participating in ADCC.
c.Both the Fc fragment and the Fab fragments of this antibody retain the ability to kill virus infected cells by participating in ADCC.
d.Neither the Fc fragment nor the Fab fragments of this antibody retain the ability to kill virus infected cells by participating in ADCC.
d.Neither the Fc fragment nor the Fab fragments of this antibody retain the ability to kill virus infected cells by participating in ADCC.
A plasma cell secretes an IgG antibody that binds to a protein from the influenza A virus. True/False: It is possible for this antibody to possess all of the following functions/abilities: To neutralize influenza virus infectivity, to function as an effective opsonizing antibody, to activate the classic complement cascade on the surface of influenza virus-infected cells, and to participate in the killing of influenza virus-infected cells through the process of ADCC.
True
A woman lacks a functional gene for the poly Ig receptor (fact). True/False: This woman would not be able to provide normal passive immunity to her fetus.
False