Describe general features and importance of B cell circulation through the body (immune-surveillance).
Features:
- phase 4 (searching for infection): Recirculation of mature B cells between lymph, blood, secondary lymphoid tissues
- phase 5 ( finding infection): Activation clonal expansion of the cells by pathogen derived antigens in secondary lymphoid tissues
- phase 6 (attacking infection): Differentiation to antibody secreting plasma cells in memory B cells in the secondary lymphoid tissue
B-cell circulation importance:
- Origination and Maturation: B cells originate from stem cells in the bone marrow. During their maturation process, they undergo genetic rearrangements to generate a diverse repertoire of B cell receptors (BCRs), which are specialized proteins on their surface that recognize specific antigens.
- Migration: Once mature, B cells leave the bone marrow and circulate through the bloodstream, lymph nodes, and lymphoid tissues such as the spleen and tonsils. They continually survey these areas for the presence of antigens.
- Recognition of Antigens: When B cells encounter antigens that match their BCRs, they become activated. Antigens are molecules, usually proteins, that are foreign to the body and can trigger an immune response.
- Activation and Differentiation: Upon activation, B cells undergo clonal expansion and differentiation. Some B cells differentiate into plasma cells, which are specialized factories for producing antibodies. Others become memory B cells, which persist for a long time and provide a rapid and robust response upon re-exposure to the same antigen.
- Antibody Production: Plasma cells secrete large quantities of antibodies, also known as immunoglobulins (Ig), into the bloodstream and surrounding tissues. Antibodies bind to antigens, marking them for destruction by other immune cells or neutralizing their harmful effects directly.
- Immune Memory: Memory B cells play a crucial role in immunological memory. They "remember" previous encounters with specific pathogens and mount a faster and more robust response upon re-exposure. This memory response is the basis for vaccination, where the immune system is primed to recognize and combat specific pathogens before they can cause disease.
- Immune Surveillance: B cell circulation throughout the body ensures continuous surveillance for invading pathogens. This ongoing monitoring helps to detect and eliminate pathogens early in the infection process, preventing or minimizing the spread of disease.
- Adaptive Response: B cells are a part of the adaptive immune response, which is highly specific to particular pathogens. This specificity allows the immune system to tailor its response to the specific threats it encounters, leading to efficient and targeted elimination of pathogens.
Overall, the circulation of B cells through the body is essential for immune surveillance, rapid response to infections, and the establishment of immunological memory, thereby playing a critical role in maintaining the body's defense against pathogens.
Describe the structural and functional properties of the B cell receptor.
What is the function of the heavy and light chain complex
(immunoglobulin),
what is the function of the Igα/Igβ dimer?
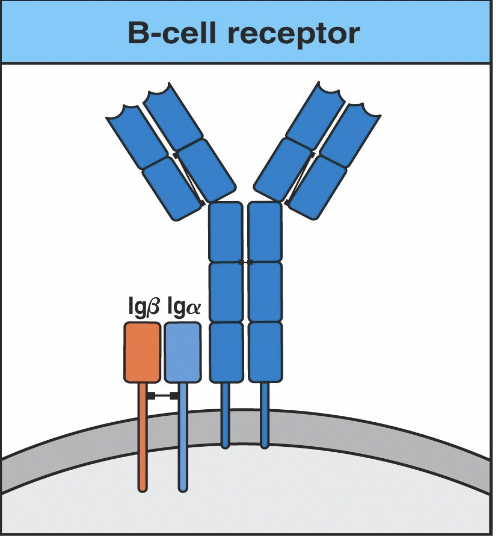
Structural and functional properties:
Function of heavy and light chain complex:
- The immunoglobulin (H + L chains) component of the BCR is responsible for antigen binding.
Function of the Igα/Igβ dimer:
- The Igα and Igβ subunits of the BCR are responsible for signal transduction.
- Structure:
- The BCR consists of membrane-bound immunoglobulin (Ig) molecules attached to the surface of B cells.
- Each BCR is composed of two identical heavy chains and two identical light chains.
- The heavy and light chains are linked by disulfide bonds and form a Y-shaped structure.
- The variable regions of both the heavy and light chains contain antigen-binding sites, which determine the specificity of the BCR for particular antigens.
- The constant regions of the heavy and light chains anchor the BCR to the B cell membrane and transduce signaling upon antigen binding.
- Function of Heavy and Light Chain
Complex (Immunoglobulin):
- The heavy and light chain complex, together forming the immunoglobulin, is responsible for antigen recognition and binding.
- The variable regions of the heavy and light chains contain hypervariable regions, also known as complementarity-determining regions (CDRs), which directly interact with antigens.
- Through somatic hypermutation and gene rearrangement processes, B cells generate a diverse repertoire of BCRs with unique antigen-binding specificities.
- Upon antigen binding, the BCR initiates intracellular signaling cascades that lead to B cell activation and subsequent immune responses.
- Function of
the Igα/Igβ Dimer:
- The Igα/Igβ dimer, also known as the B cell co-receptor, is associated with the BCR complex and assists in signal transduction upon antigen binding.
- Igα and Igβ are transmembrane proteins that form a heterodimer on the cytoplasmic side of the B cell membrane.
- The Igα/Igβ complex functions as a signal amplifier, enhancing the signaling strength and efficiency of the BCR upon antigen engagement.
- It interacts with intracellular signaling molecules, such as Igα/Igβ-associated protein kinases, to transmit signals that ultimately lead to B cell activation, proliferation, and differentiation.
- The Igα/Igβ dimer is crucial for proper B cell development, antigen recognition, and initiation of adaptive immune responses.
In summary, the B cell receptor is composed of membrane-bound immunoglobulin molecules (heavy and light chain complex) responsible for antigen recognition and binding. The Igα/Igβ dimer acts as a co-receptor, aiding in signal transduction and amplification upon antigen engagement, thus facilitating B cell activation and immune responses.
TD Antigen
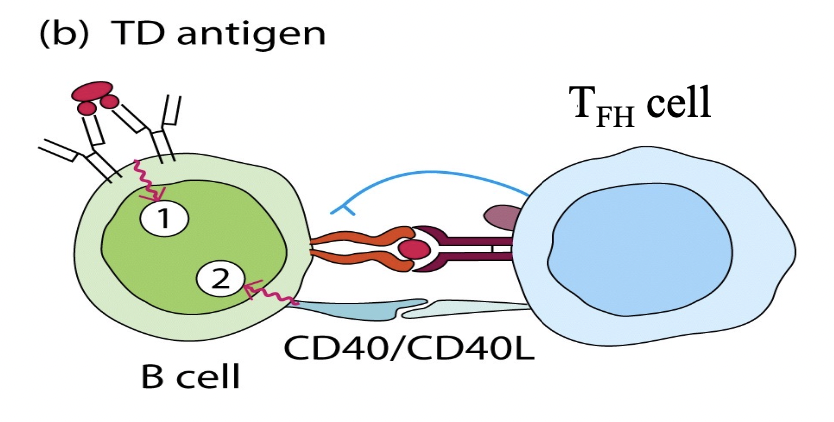
Describe the general difference between a T-dependent and a
T-independent antigen with respect to their abilities to stimulate
a
naïve B cell.
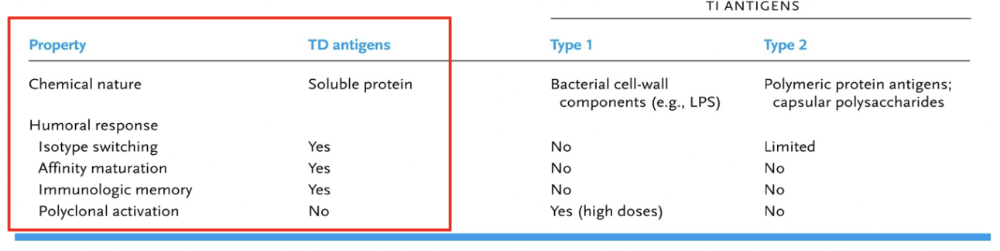
In general 2 distinct signals are required to induce B cell activation
T-dependent (TD):
- Thymus dependent
- The most useful B cell responses occur in response to TD antigen
- There is T cell assistance for this cell
T-Independent (TI):
- Thymus independent
- Stimulate itself by itself with no T cell assistance
* The DIFFERENCES are Important (The red box). We want these things to occur for immune response.
- T-Dependent Antigens:
- T-dependent antigens are typically large, complex molecules such as proteins or polysaccharides.
- Naïve B cells require assistance from T helper (Th) cells to mount an effective immune response against T-dependent antigens.
- Upon encountering a T-dependent antigen, a naïve B cell internalizes and processes the antigen, presenting peptide fragments on its surface in association with major histocompatibility complex class II (MHC-II) molecules.
- The B cell then interacts with Th cells that recognize the same antigen presented by antigen-presenting cells (APCs) and receive co-stimulatory signals from the Th cells.
- This interaction triggers the activation and differentiation of the naïve B cell into plasma cells, which secrete high-affinity antibodies specific to the antigen.
- T-dependent immune responses generate memory B cells, providing long-term immunity upon re-exposure to the antigen.
- T-Independent Antigens:
- T-independent antigens are typically repetitive, highly ordered structures such as certain bacterial polysaccharides or lipopolysaccharides (LPS).
- Naïve B cells can directly recognize and respond to T-independent antigens without the need for assistance from T cells.
- T-independent antigens can cross-link multiple BCRs simultaneously, leading to robust B cell activation and proliferation.
- However, the immune response elicited by T-independent antigens is often less robust and of shorter duration compared to T-dependent responses.
- T-independent immune responses typically do not generate significant numbers of memory B cells, resulting in weaker long-term immunity upon re-exposure to the antigen.
In summary, the main difference between T-dependent and T-independent antigens regarding their abilities to stimulate naïve B cells lies in the requirement for T cell assistance and the strength and duration of the resulting immune response. T-dependent antigens require interaction with Th cells for optimal B cell activation and generate stronger and more sustained immune responses, including the production of memory B cells. In contrast, T-independent antigens can directly activate B cells but typically result in weaker and shorter-lived immune responses with limited memory cell generation.
Describe the purpose of the CD40/CD40L interaction. What is achieved by this binding interaction?
- Purpose:
- CD40 (cluster of differentiation 40) is a cell surface receptor primarily expressed on B cells, but also on other antigen-presenting cells (APCs) such as dendritic cells and macrophages.
- CD40L (CD40 ligand), also known as CD154, is a protein primarily expressed on activated CD4+ T cells (helper T cells).
- Binding Interaction:
- The CD40/CD40L interaction occurs when CD40 on the surface of B cells binds to CD40L expressed on activated CD4+ T cells.
- This binding interaction is a crucial step in the activation of B cells during T-dependent immune responses.
- Achievements:
- Activation Signal: The CD40/CD40L interaction provides a co-stimulatory signal to the B cell, which is required for its full activation. This interaction, along with antigen recognition by the B cell receptor (BCR), ensures proper B cell activation and differentiation.
- Proliferation and Differentiation: Upon receiving signals from CD40 engagement, B cells undergo proliferation and differentiation. They differentiate into plasma cells, which are specialized for antibody production, and memory B cells, which provide long-term immunity upon re-exposure to the same antigen.
- Antibody Class Switching: CD40 engagement also plays a critical role in antibody class switching, where B cells switch the class of antibodies they produce from IgM to other antibody isotypes such as IgG, IgA, or IgE. This process allows B cells to tailor the immune response to the type of pathogen encountered.
- Germinal Center Formation: The CD40/CD40L interaction contributes to the formation and maintenance of germinal centers within secondary lymphoid organs, such as lymph nodes and the spleen. Germinal centers are specialized microenvironments where B cells undergo affinity maturation, somatic hypermutation, and selection, leading to the generation of high-affinity antibodies.
- Long-Term Immunity: By promoting B cell activation, proliferation, differentiation, and antibody class switching, the CD40/CD40L interaction ultimately contributes to the generation of long-term immunity against pathogens. Memory B cells generated during T-dependent immune responses can rapidly respond to re-exposure to the same antigen, leading to a faster and more robust immune response.
In summary, the CD40/CD40L interaction is essential for effective B cell activation and the generation of robust and long-lasting immune responses mediated by B cells, including the production of high-affinity antibodies and the establishment of immunological memory.
Explain why it is important for a B cell to express MHC-II and B7.
Describe the types of cell to cell communication that
occurs
between a naïve B cell and an effector TFH cell.
MHC-II and B7 importance:
-
- MHC-II Expression:
- MHC-II molecules are responsible for presenting antigenic peptides to CD4+ helper T cells.
- When a B cell encounters an antigen and internalizes it, the antigenic peptides derived from the antigen are loaded onto MHC-II molecules within the B cell.
- MHC-II/peptide complexes are then expressed on the surface of the B cell, where they can be recognized by CD4+ T cells.
- This interaction between MHC-II/peptide complexes on the B cell and the T cell receptor (TCR) on the CD4+ T cell is crucial for initiating T cell activation and subsequent help to the B cell.
- B7 Expression:
- B7 molecules, specifically B7-1 (CD80) and B7-2 (CD86), serve as co-stimulatory molecules on antigen-presenting cells (APCs) including B cells.
- When a B cell is activated by an antigen, it upregulates the expression of B7 molecules.
- B7 molecules on the B cell interact with CD28 receptors on the surface of CD4+ helper T cells.
- This B7-CD28 interaction provides a necessary co-stimulatory signal to the T cell, along with TCR engagement with MHC-II/peptide complexes, leading to T cell activation and subsequent help to the B cell.
- MHC-II Expression:
Cell:cell communication
-
- Cytokine Signaling:
- TFH cells secrete cytokines such as interleukin-21 (IL-21), which can stimulate naïve B cells.
- IL-21 promotes B cell proliferation, differentiation into plasma cells, and antibody production.
- CD40/CD40L Interaction:
- As described earlier, TFH cells express CD40 ligand (CD40L), which binds to CD40 receptors on B cells.
- This CD40/CD40L interaction provides co-stimulatory signals to B cells, enhancing their activation, proliferation, and differentiation into antibody-secreting plasma cells.
- B Cell Receptor (BCR)
Cross-Linking:
- TFH cells can indirectly influence B cell activation by promoting cross-linking of the BCR through the secretion of cytokines or via interactions with follicular dendritic cells (FDCs).
- Cross-linking of the BCR by antigen or antigen-antibody complexes enhances B cell activation and antigen presentation to TFH cells.
- Cytokine Signaling:
Describe the source of low affinity IgM produced early after B cell stimulation.
Describe the different processes that occur within
the
different regions of the lymph node (medullary cords, T cell area,
primary follicle, germinal center).
low affinity IgM:
-
-
Extrafollicular Response:
- Upon encountering an antigen, naïve B cells can undergo rapid activation outside of the germinal centers, in areas known as the extrafollicular regions of the lymphoid tissue.
- In these extrafollicular regions, B cells differentiate into short-lived plasma cells that predominantly secrete low-affinity IgM antibodies.
- This early production of low-affinity IgM provides an immediate but less specific defense against pathogens before the germinal center response matures.
-
Extrafollicular Response:
different processes:
- Dark zone: Central Blast(it is a mature cell; a dividing cell). There job is to divide and this causes somatic hypermutation. They do not express their antibodies.
- Light zone: centrocytes are re-expresssing their antibodies
- Regarding
the different processes that occur within the different regions of
the lymph node (medullary cords, T cell area, primary follicle,
germinal center):
- Medullary Cords:
- Medullary cords are located in the medulla of the lymph node.
- They contain plasma cells, which are the terminally differentiated form of B cells that produce antibodies.
- Plasma cells in the medullary cords continuously secrete antibodies into the bloodstream to provide systemic immunity.
- T Cell Area (Paracortex):
- The T cell area, also known as the paracortex, is situated between the medullary cords and the B cell follicles.
- It is primarily populated by T cells, particularly CD4+ helper T cells and CD8+ cytotoxic T cells.
- Antigen-presenting cells (APCs), such as dendritic cells and macrophages, present antigens to T cells in the paracortex, initiating T cell activation and differentiation.
- Primary Follicle:
- Primary follicles are areas within the lymph node where naïve B cells reside.
- Within primary follicles, B cells are relatively quiescent and have not yet encountered their cognate antigens.
- Follicular dendritic cells (FDCs) present within the primary follicle help to maintain B cell survival and integrity of the follicular structure.
- Germinal Center:
- Germinal centers develop within secondary follicles (follicles that have encountered antigens).
- They are specialized microenvironments where B cell activation, proliferation, and differentiation occur.
- Within the germinal center, B cells undergo somatic hypermutation, leading to the generation of B cell clones with increased affinity for the antigen.
- B cells also undergo class switching, where they switch the type of antibody they produce (e.g., from IgM to IgG).
- Germinal centers are sites of intense B cell-T cell interactions, mediated by follicular helper T cells (TFH), and are essential for the generation of high-affinity antibodies and the development of immunological memory.
- Medullary Cords:
Explain how proteins generated in the complement cascades (namely C3b and its cleavage products such as C3d) can contribute to B cell stimulation/activation.
Explain the roles of complement proteins and complement receptors in this process
- Opsonization:
- C3b is an opsonin, meaning it can bind to the surface of pathogens and mark them for phagocytosis by immune cells such as macrophages and neutrophils.
- When C3b binds to the surface of a pathogen, it enhances the recognition and uptake of the pathogen by phagocytic cells, thus promoting the clearance of the pathogen from the body.
- This opsonization process helps to eliminate pathogens more efficiently and also indirectly contributes to B cell activation.
- B Cell Stimulation/Activation:
- C3d, a cleavage product of C3b, plays a specific role in B cell stimulation and activation.
- When C3b covalently binds to antigens on the surface of pathogens, it undergoes further proteolytic cleavage to generate C3d fragments.
- C3d fragments bound to antigens can act as co-stimulatory molecules for B cells during antigen presentation.
- B cells express complement receptors, particularly CR2 (complement receptor 2, also known as CD21), which specifically bind to C3d fragments on the surface of antigens.
- The binding of C3d to CR2 on B cells enhances B cell activation by providing an additional co-stimulatory signal along with antigen recognition by the B cell receptor (BCR).
- This co-stimulation promotes B cell proliferation, differentiation, and antibody production in response to the antigen.
- Roles
of Complement Proteins and Complement Receptors:
- Complement proteins, including C3b and its cleavage products, serve as molecular tags that enhance the recognition and elimination of pathogens by the immune system.
- Complement receptors, such as CR2 (CD21) on B cells, recognize and bind to complement fragments, facilitating interactions between B cells and complement-coated antigens.
- The interaction between complement fragments and complement receptors provides additional signals to B cells, augmenting their activation and amplifying the immune response against pathogens.
In summary, proteins generated in the complement cascades, such as C3b and its cleavage products like C3d, contribute to B cell stimulation and activation by promoting opsonization of pathogens and by acting as co-stimulatory molecules for B cells during antigen presentation. Complement receptors on B cells recognize complement fragments on the surface of antigens, enhancing B cell activation and the subsequent immune response.
Describe the role of the follicular dendritic cell in the processes of B cell selection and affinity maturation.
- B cells (called centrocytes at this point) interact with antigen on the surface of follicular dendritic cells. Centrocytes compete for antigen binding and T cell help.
- Class switching and development into plasma cells is influenced by specific cytokines (released from TFH cells).
Picture of process on slide 12
Follicular dendritic cells (FDCs) play critical roles in the processes of B cell selection and affinity maturation within the germinal centers of secondary lymphoid organs such as lymph nodes and spleen. Here's how FDCs contribute to these processes:
- B Cell Selection:
- Within the germinal center, FDCs create a specialized microenvironment where B cells undergo selection based on their antigen specificity and affinity.
- FDCs capture and retain intact antigen-antibody complexes on their surface, forming immune complexes.
- These immune complexes are derived from antigens that have been opsonized with complement proteins, such as C3d, or bound by antibodies secreted by B cells.
- B cells with B cell receptors (BCRs) that have high affinity for the antigen are more likely to capture and retain antigen-antibody complexes on the FDCs compared to B cells with lower affinity BCRs.
- This process effectively selects B cells with higher affinity BCRs for further proliferation and differentiation, promoting the generation of antibodies with improved antigen-binding capabilities.
- Affinity Maturation:
- Affinity maturation is the process by which B cells undergo somatic hypermutation and selection to produce antibodies with increased affinity for the antigen.
- FDCs provide a crucial environment for affinity maturation to occur within the germinal center.
- B cells interact with FDC-bound immune complexes through their BCRs, presenting antigen to follicular helper T cells (TFH).
- TFH cells provide signals to the B cells, including cytokines such as interleukin-21 (IL-21), which promote B cell proliferation and somatic hypermutation.
- B cells with mutations that result in increased affinity for the antigen are more likely to capture and retain antigen-antibody complexes on the FDCs.
- Over successive rounds of proliferation, mutation, and selection, B cells with higher affinity BCRs outcompete those with lower affinity BCRs, leading to the production of antibodies with improved antigen-binding affinity.
In summary, follicular dendritic cells play crucial roles in B cell selection and affinity maturation within germinal centers. By capturing and retaining antigen-antibody complexes on their surface, FDCs create a microenvironment where B cells with high-affinity BCRs are selected for further proliferation and differentiation. Additionally, FDCs provide a platform for B cells to interact with TFH cells and undergo somatic hypermutation, leading to the generation of antibodies with increased affinity for the antigen.
Explain the general role
that specific cytokines and Tfh cells play in the processes
of B cell stimulation, B cell entry into the cell
cycle,
class-switching, differentiation into memory B cells and plasma cells.
General role:
Specific cytokines and T follicular helper (Tfh) cells play essential roles in various aspects of B cell stimulation, proliferation, differentiation, and antibody production. Here's an overview of their general roles in these processes:
- B Cell Stimulation:
- Cytokines secreted by Tfh cells, particularly interleukin-21 (IL-21), play a central role in stimulating B cell activation and proliferation.
- IL-21 acts directly on B cells to promote their survival, proliferation, and differentiation into antibody-secreting cells.
- B Cell Entry into the Cell Cycle:
- IL-21, along with other cytokines produced by Tfh cells, stimulates B cells to enter the cell cycle and undergo rapid proliferation.
- B cells that receive signals from Tfh cells and cytokines progress from the G0 phase (quiescent phase) of the cell cycle into the G1 phase, initiating cell division.
- Class-Switching:
- Cytokines secreted by Tfh cells, such as IL-4 and transforming growth factor-beta (TGF-β), play crucial roles in promoting class-switching of antibodies.
- IL-4 induces class-switch recombination (CSR) in B cells, leading to the production of antibodies of different isotypes (e.g., IgG, IgA, IgE) with distinct effector functions.
- TGF-β synergizes with IL-4 to enhance class-switching to IgA, particularly in mucosal tissues.
- Differentiation into Memory
B Cells and Plasma Cells:
- Tfh cells provide signals to B cells that promote their differentiation into memory B cells and plasma cells.
- IL-21, along with other cytokines produced by Tfh cells, promotes the differentiation of activated B cells into memory B cells.
- Plasma cell differentiation is induced by a combination of signals from Tfh cells, including IL-21, IL-4, and other cytokines.
- B cells that receive appropriate signals differentiate into plasma cells, which are specialized for the production and secretion of antibodies.
In summary, specific cytokines secreted by Tfh cells, particularly IL-21, along with other cytokines such as IL-4 and TGF-β, play critical roles in B cell stimulation, proliferation, class-switching, and differentiation into memory B cells and plasma cells. These interactions between Tfh cells and B cells are essential for the generation of effective humoral immune responses against pathogens.
Monoclonal vs polyclonal
Monoclonal: Only One
Polyclonal Activation: most of the antibody you create are not good
In summary, monoclonal antibodies are produced by identical immune cells and recognize a single epitope with high specificity, whereas polyclonal antibodies are produced by multiple different immune cells and recognize multiple epitopes on a target antigen, resulting in broader specificity. Both monoclonal and polyclonal antibodies have unique applications depending on their specificities, affinities, and intended uses in research, diagnostics, and therapeutics.
Supercharge Signal 1
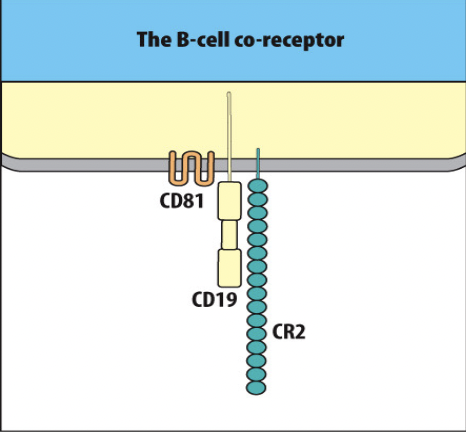
- B cell activation can be enhanced by signaling through the B cell co-receptor (CR2), which binds to the complement fragment C3d.
naive bcell vs. mature bcell
Naive B cell = naive bcell
Mature B cell= plasma cell or memory cell
True/False: Most of the processes that will be discussed today are more likely to occur in the bone marrow than in the spleen.
False

The nude mouse lacks a functional FoxN1 gene which results in the failure to develop a thymus (and in the failure to develop normal hair follicles). True/False : The nude mouse is not able to make normal antibody responses following natural infection or vaccination.
True
Which one of the following defects in the complement system would result in the most significant decrease in B cell responses to foreign antigens?
Which one of the following defects in the complement system would result in the most significant decrease in B cell responses to foreign antigens?
a. Lack of a functional C9 protein.
b. Inability to produce any form of the C5 convertase enzyme.
c. Lack of a functional factor B protein.
d. Inability to produce any form of a C3 convertase enzyme.
e. Lack of a functional factor H protein.
d. Inability to produce any form of a C3 convertase enzyme.
True/False: A B cell that displays decreased affinity for its antigen as a result of somatic hypermutation in the germinal center has a decreased chance of receiving signal #1 from antigen on a follicular dendritic cell and a decreased chance of receiving signal #2 from a TH cell.
True
tophat
...
tophat
...
tophat
...
tophat
...
tophat
...