A student ran the following reaction in the laboratory at
450 K:
PCl5(g)
PCl3(g) +
Cl2(g)
When she introduced 1.07 moles of
PCl5(g) into a 1.00 liter container, she
found the equilibrium concentration of
Cl2(g) to be 3.65E-2 M.
Calculate the equilibrium constant, Kc, she obtained
for this reaction
1.29E-3
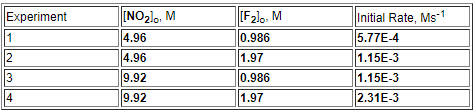
The following initial rate data are for the reaction of
nitrogen dioxide with fluorine:
2 NO2 + F2 2 NO2F
Complete the rate law for this reaction in the box
below.
Use the form
k[A]m[B]n , where '1'
is understood for m or n and concentrations taken to the
zero power do not appear. Don't enter 1 for
m or n
k[NO_2_][F_2_]
1.18E-4
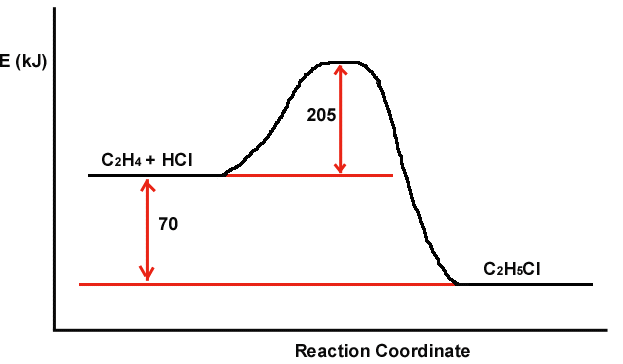
A reaction profile (not to scale!) for the reaction
C2H4 + HCl
C2H5Cl is shown below:
Which of the following are true?
Choose all that apply.
a. If the energy of the activated complex were increased,
Ea would increase.
b. The value of Ea at higher temperatures would be lower than 205 kJ.
c.The energy of the products is lower than the energy of the reactants.
d.The reaction is exothermic.
a b d
Consider the following system at equilibrium where Ho =
-16.1 kJ, and Kc = 154,
at 298 K:
2 NO(g) +
Br2(g) 2 NOBr(g)
If the VOLUME of the equilibrium system is
suddenly increased at constant temperature:
The value of Kc
C
A. Increases.B. Decreases.C. Remains the same.
The value of Q
A. Is greater than K.B. Is equal to K.C. Is less than K.
The reaction must
A. Run in the forward direction to restablish equilibrium.B. Run in
the reverse direction to restablish equilibrium.C. Remain the same.
Already at equilibrium.
The number of moles of Br2 will
A. Increase.B. Decrease.C. Remain the same.
C
A
B
A
The gas phase reaction between hydrogen and iodine is proposed to
occur as follows:
.....step 1.....
fast:...... I2
2 I
.....step 2.....
slow:.... H2 + 2 I
2 HI
(1) What is the equation for the overall reaction?
Use the smallest integer coefficients possible. If a box is not
needed, leave it blank.
(2) Enter the formula of any species that acts as a reaction intermediate? If none leave box blank:
(3) Complete the rate law for the
overall reaction that is consistent with this mechanism.
Use the
form k[A]m[B]n... , where '1'
is understood (so don't write it if it's a '1') for
m, n etc.
1) H2+I2 -> 2HI
2) I
3) k[H2][I2]
Hydrogen peroxide decomposes into water and oxygen in a first-order
process.
H2O2(aq) →
H2O() + 1/2 O2(g)
At 20.0 °C, the
half-life for the reaction is 3.92E+4 seconds. If the initial
concentration of hydrogen peroxide is 0.822 M, what
is the concentration after 3.27 days?
5.56E-3
The equilibrium constant, Kc, for the following reaction
is 77.5 at 600 K.
CO(g) +
Cl2(g)
COCl2(g)
Assuming that you start with
equal concentrations of CO and
Cl2 and no
COCl2 is initially present, describe the
relative abundance of each species present at equilibrium.
[CO]
[COCl2]
[Cl2]
1)
Higher 2)
Lower 3)
Can't tell
2
1
2
Consider the reaction:
C(s) + 1/2 O2(g) CO(g)
Write the equilibrium constant for this reaction in terms of the
equilibrium constants, Ka and
Kb, for reactions a and
b below:
a.)
C(s) + O2(g)
CO2(g).............Ka
b.) CO(g) + 1/2 O2(g) CO2(g).............Kb
Ka / Kb
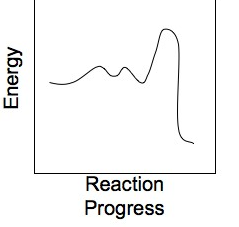
For the reaction coordinate diagram shown below, what is the order of the reaction?
two fast steps followed by one slow step, with a build up of intermediates between all three steps
onsider the following system at equilibrium where Kc =
1.29E-2 and Ho = 108
kJ/mol at 600 K:
COCl2(g)
CO(g) +
Cl2(g)
The production of
CO(g) is favored by
Indicate True (T) or False (F)
for each of the following choices.
1. Increasing the
temperature.
2
. Decreasing the pressure (by
changing the volume).
3.
Decreasing the volume.
4.
Removing COCl2.
5. Adding Cl2.
T
T
F
F
F
For the reaction A → B, the rate law is
.
What are the
units of the rate constant where time is measured in seconds?
1/s
The activation energy for the gas phase
decomposition of t-butyl acetate is
170 kJ.
CH3COOC(CH3)3
(CH3)2C=CH2 + CH3COOH
The rate constant at 539 K is
7.12E-4 /s. The rate constant will be
8.33E-3 /s at
576
The equilibrium constant, Kc, for the following reaction
is 1.29E-2 at 600 K.
Calculate
Kp for this reaction at this temperature.
COCl2(g)
CO(g) +
Cl2(g)
0.635
Which of the following statements is/are CORRECT?
1.A reaction
will proceed in the forward direct when Q>K
2.A reaction favors the formation of products if K >> 1.
3.In an Endothermic reaction, increasing the temperature will increase [Reactants
2 only
For the decomposition of ammonia on a tungsten surface at 1100 oC
2 NH3 -> N2 + 3 H2
the average rate of disappearance of NH3
over the time period from t = 0 s to
t = 1.63E+3 s is found to be
3.40E-6 M s
-1.
What is the average rate of appearance of
N2 over the same time period?
1.7E-6
Which of the following is true regarding the rate constant (k)?
1. It is temperature independent
2. It is concentration
independent
3. It is a proportionality constant
2 and 3
Write the equilibrium constant expression, K, for the following
reaction:
Please enter the compounds in the order given in the
reaction.If either the numerator or denominator is 1, please enter 1.
BaSO4(s)
Ba2+(aq) +
SO4
2-(aq)
[Ba2+] [SO42-]/1
[Ba2+][SO4 2-]/1
The equilibrium constant, Kc, for the following reaction
is 55.6 at 698 K:
H2(g) +
I2(g) 2 HI(g)
Calculate the equilibrium concentrations of reactants and
product when 0.305 moles of
H2 and 0.305 moles of
I2 are introduced into a 1.00 L vessel at
698 K.
H2: 0.0645
I2: 0.0645
HI: 0.4810
Consider the following reaction where Kp =
9.52E-2 at 350 K:
CH4(g) +
CCl4(g) 2 CH2Cl2(g)
If the three gases are mixed in a rigid container at
350 K so that the partial pressure of each gas is
initially one atm, what will happen?
Indicate True (T) or False (F)
for each of the following:
1
. A reaction will occur in which
CH2Cl2(g) is
produced.
2. Kp
will decrease.
3. A reaction
will occur in which CH4 is
produced.
4. Q is
greater than K.
5. The
reaction is at equilibrium. No further reaction will occur.
F
F
T
T
F
or the gas phase decomposition of t-butyl acetate,
CH3COOC(CH3)3
(CH3)2C=CH2 + CH3COOH
the rate constant has been determined at several temperatures.
When ln k in s-1 is plotted against the reciprocal of the
Kelvin temperature, the resulting linear plot has a slope of
-2.04E+4 K and a y-intercept of 30.7.
The activation energy for the gas phase
decomposition of t-butyl acetate is
170
NaHCO3
(aq) + HNO3
(aq) NaNO3(aq) + H2O(l)
+ CO2(g)
Which of the following could be a way to increase the rate of
the reaction above?
Indicate "yes" or "no"
for each choice.
Increasing the temperature.
Doubling the pressure.
Increasing the concentration of NaHCO3
Increasing the concentration of HNO3
Yes
No
Yes
Yes
The reaction of hypochlorite ion with iodide ion in 1 M aqueous
hydroxide solution
OCl- + I- ->
OI- + Cl-
is first order in
OCl- and first order in I-.
Complete the rate law for this reaction in the box
below.
Use the form
k[A]m[B]n... , where '1' is
understood for m, n ...(don't enter 1) and
concentrations taken to the zero power do not appear
k[OCl-][I-]
Hydrogen peroxide decomposes into water and oxygen in a first-order
process.
H2O2(aq) →
H2O() + 1/2 O2(g)
At 20.0 °C, the
half-life for the reaction is 3.92E+4 seconds. If the initial
concentration of hydrogen peroxide is 0.696 M, what
is the concentration after 7.15 days?
1.25E-5
CO (g) +
Br2 (g)
COBr2(g)
Which of the following could be a way to increase the rate of
the reaction indicated above?
Indicate "yes" or
"no" for each choice.
Increasing the temperature.
Doubling the pressure.
Increasing the concentration of
CO.
Increasing the concentration of Br2.
Yes to all
In a study of the decomposition of nitrosyl bromide at 10 oC
NOBr NO + ½ Br2
the concentration of NOBr was followed as a
function of time.
It was found that a graph of 1/[NOBr] versus
time in seconds gave a straight line with a slope of
1.22 M-1s-1
and a y-intercept of 2.60 M-1.
Based on this plot, the reaction is
____ order in NOBr and the rate
constant for the reaction is ____
second
1.22
For the second-order reaction below, the rate constant of the
reaction is 8.88E-3 M–1s–1. How
long (in seconds) is required to decrease the concentration of A from
1.34 M to 0.119 M?
2A → B rate = k [A]2
862
The equilibrium constant, Kp, for the following reaction
is 0.497 at 500 K.
Calculate
Kc for this reaction at this temperature.
PCl5(g)
PCl3(g) +
Cl2(g)
1.21E-2
The activation energy for the gas phase
isomerization of dimethyl maleate is
111 kJ/mol.
cis-CH3OOCCH=CHCOOCH3 trans-CH3OOCCH=CHCOOCH3
The rate constant is 7.08E-4 s-1 at
701 K. At what temperature is the rate constant
3.77E-3 s-1?
768
For the gas phase decomposition of vinyl
ethyl ether,
CH2=CH-OC2H5
C2H4 + CH3CHO
the rate constant has been determined at several temperatures.
When ln k in s-1 is plotted against the reciprocal of the
Kelvin temperature, the resulting linear plot has a slope of
-2.20E+4 K and a y-intercept of 26.3.
The activation energy for the gas phase
decomposition of vinyl ethyl ether is
-183.2
Consider the following reaction:
NH4Cl (s) -> NH3 (g) + HCl (g)
If a flask maintained at 554 K contains 0.125 moles of NH4Cl(s) in equilibrium with 1.59E-2 M NH3(g) and 2.73E-2 M HCl(g), what is the value of the equilbrium constant at 554 K?
4.34E-4
What does decreasing volume do?
Increases pressure
goes towards fewer moles
if Q > K
increase products
if Q < K
increase reactants