Why is Cl2 non-selective?
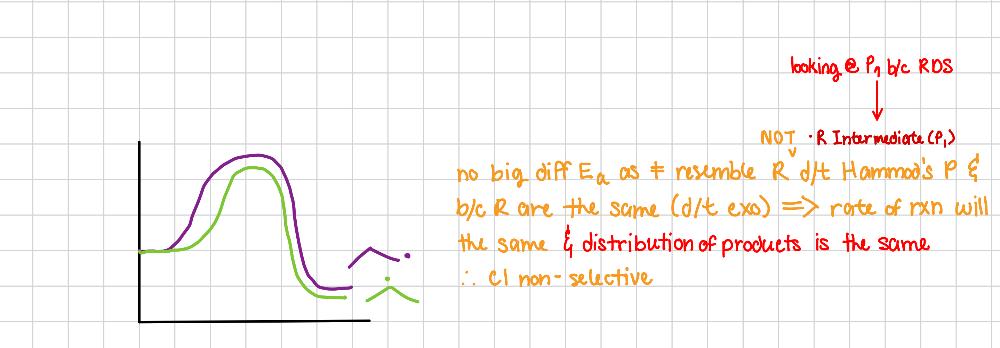
Why is Br2 selective?
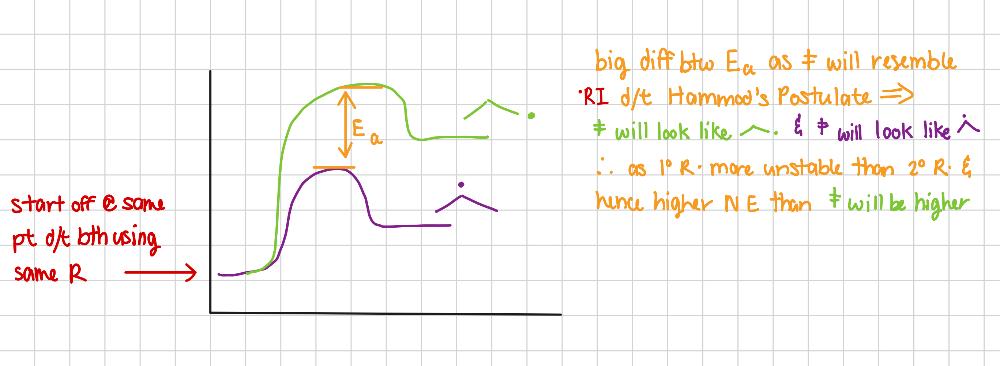
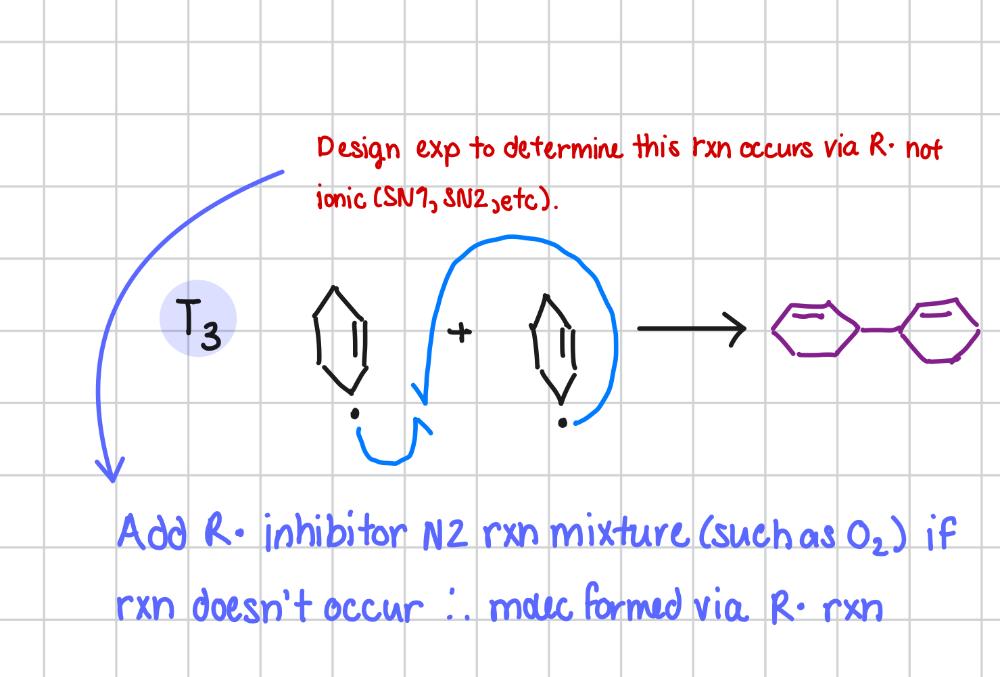
Design an exp to determine if a rxn occurs via radicals not ionic (SN1, SN2, etc).
What is the order of importance in which structures contribute to the hybrid

What is resonance?
e- delocalization through conjugated systems (need p orbitals)
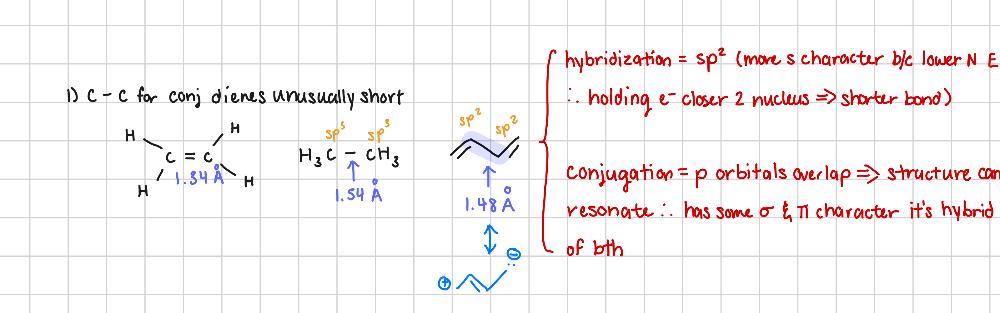
Why is the length of the C-C for conjugated dienes unusually short? (2 arguments)
Completely conjugated →___ λmax ∴ absorbs ___ wavelength (UV light)
high; greater
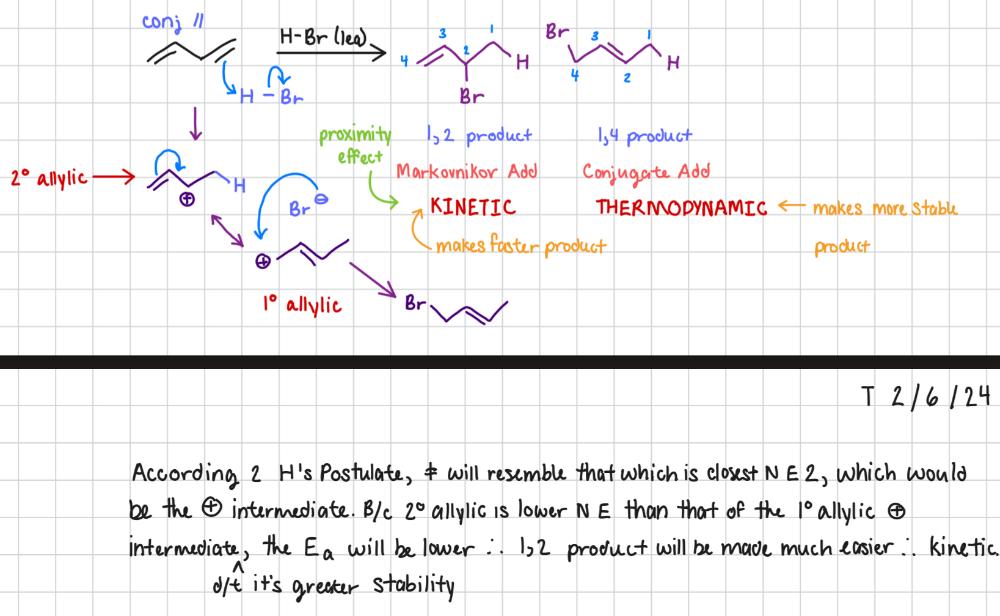
Why is the kinetic product faster than the thermodynamic product?
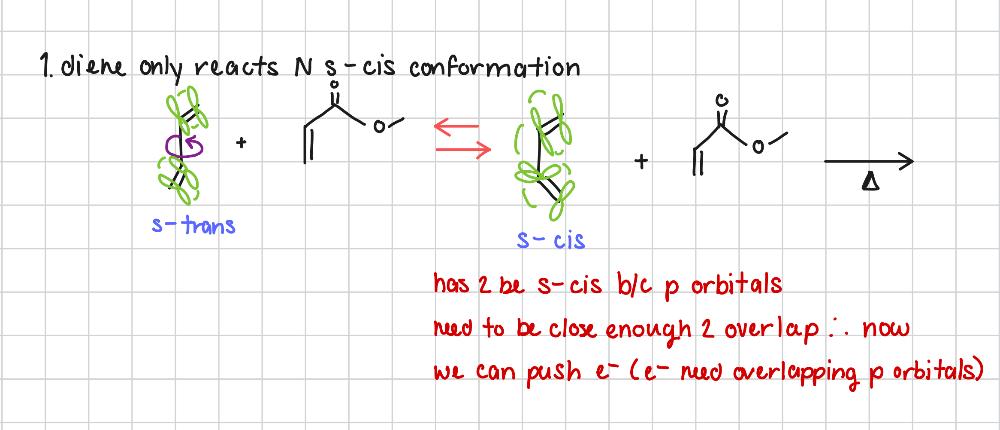
For Diels Alder rxns, why does the diene have to be in s-cis conformation?

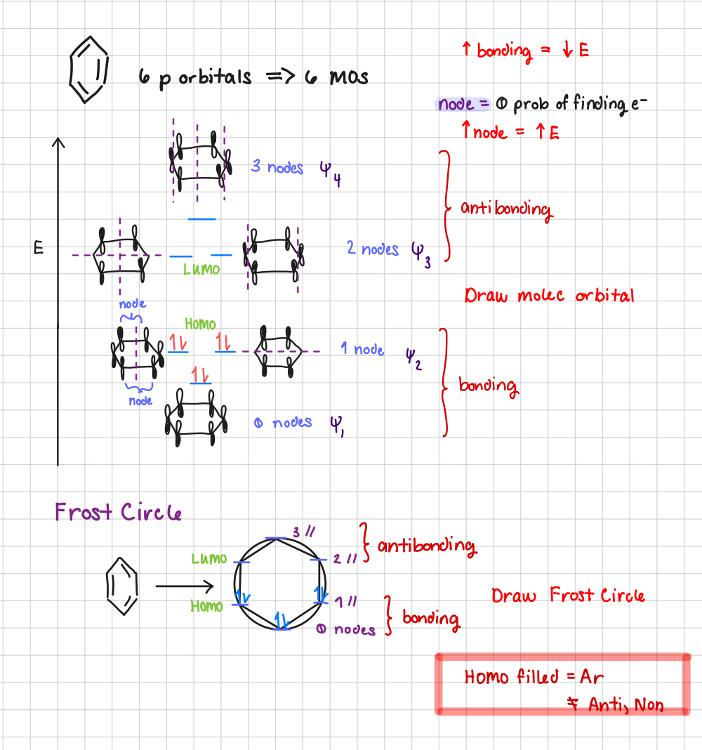
Draw MOs & Frost Circle for the benzene.
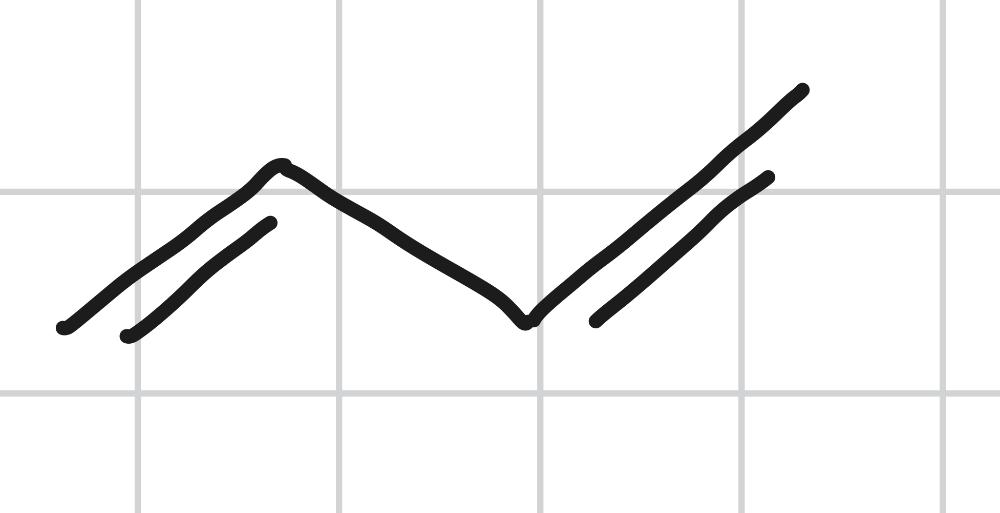
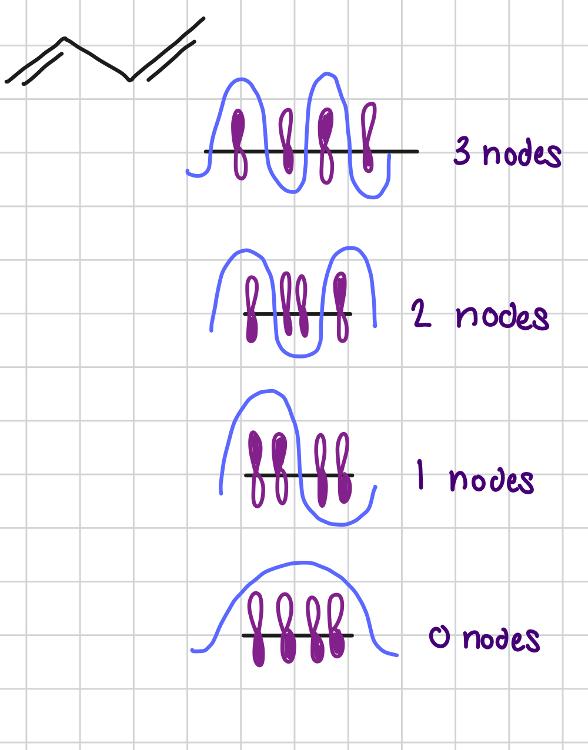
Draw MOs for 1,3 - butadiene.
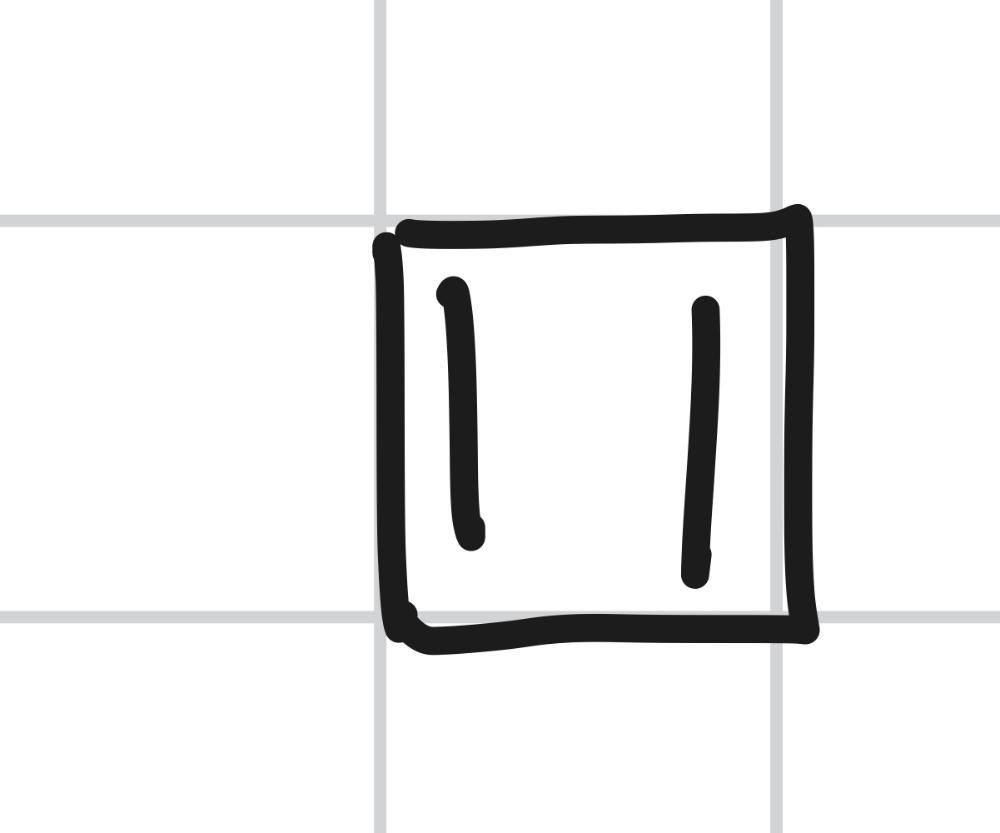
Draw Frost Circle
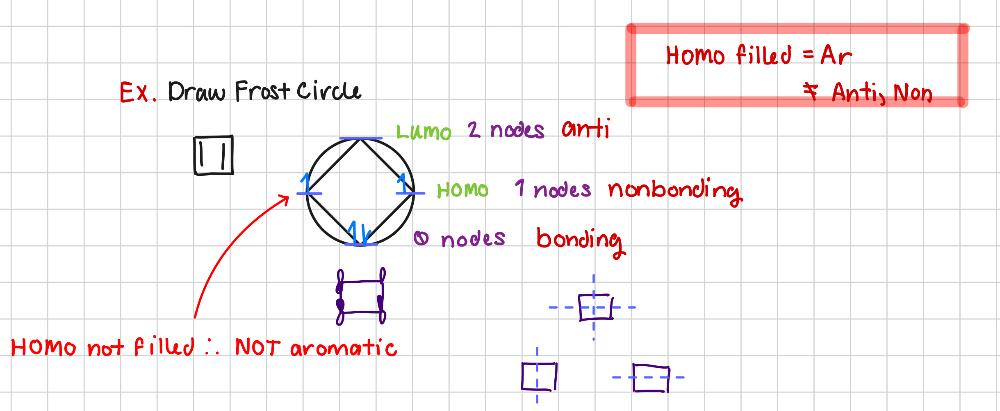
Why is breaking Ar the RDS?
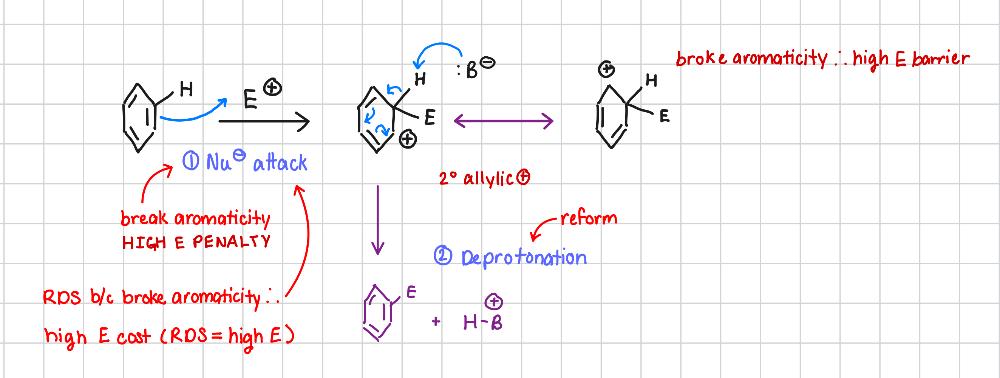
ELECTRONIC ARGUMENT
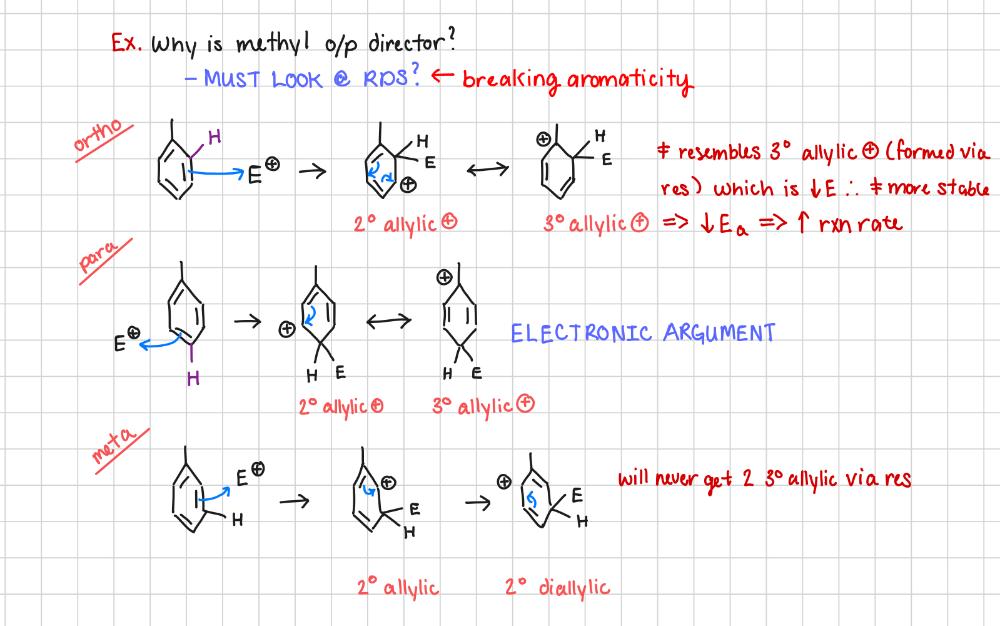
Why is methyl an o/p director?
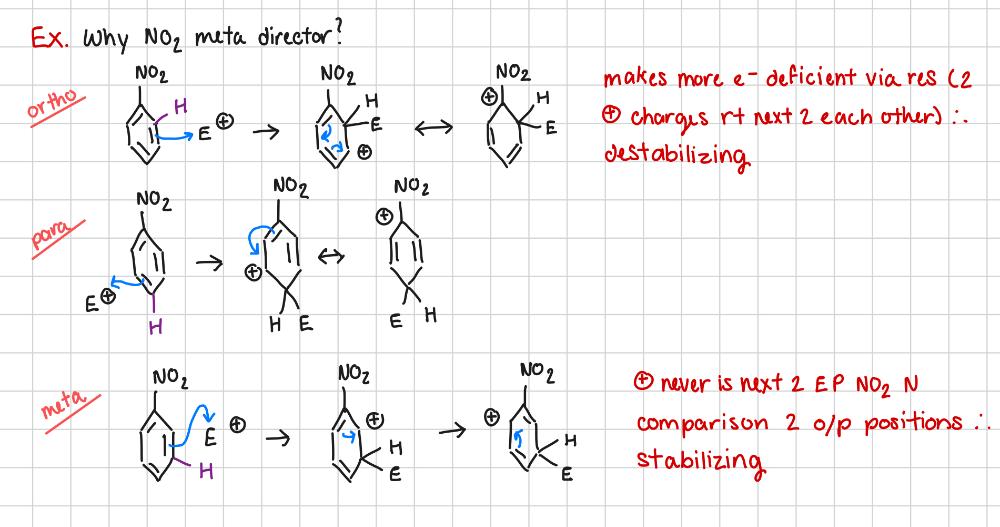
Why is NO2 a meta director?