1
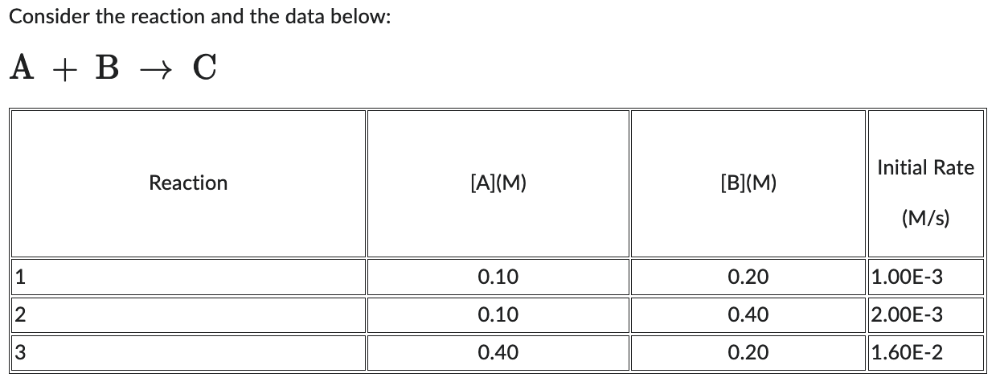
The order with respect to A is:
2
(Rate 3 / Rate 1) = (k [A]3 m [B]3 n / k [A]1 m [B]1 n)
(1.60E-2 / 1.00E-3) = (k [0.40]3 m [0.20]3 n / k [0.10]1 m [0.20]1 n)
(1.60E-2 / 1.00E-3) = (k [0.40]3 m [0.20]3 n / k [0.10]1 m [0.20]1 n)
(1.60E-2 / 1.00E-3) = ([0.40]3 m / [0.10]1 m)
16 = 4m
m = ( ln(16) / ln(4) )
m = 2
2
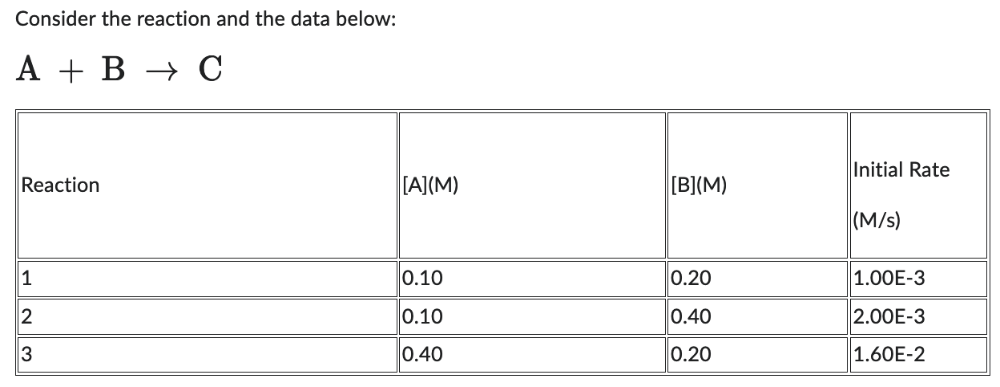
The value closest to k is:
0.1 OR 1
3
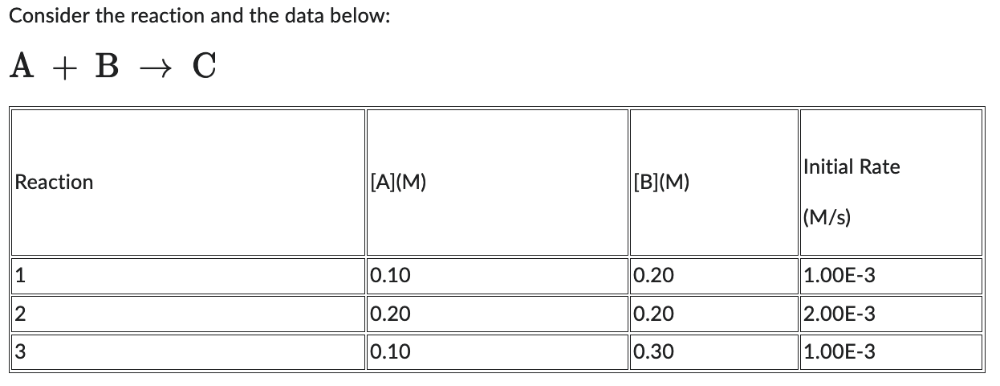
The order with respect to A is:
2 = 2
1
4
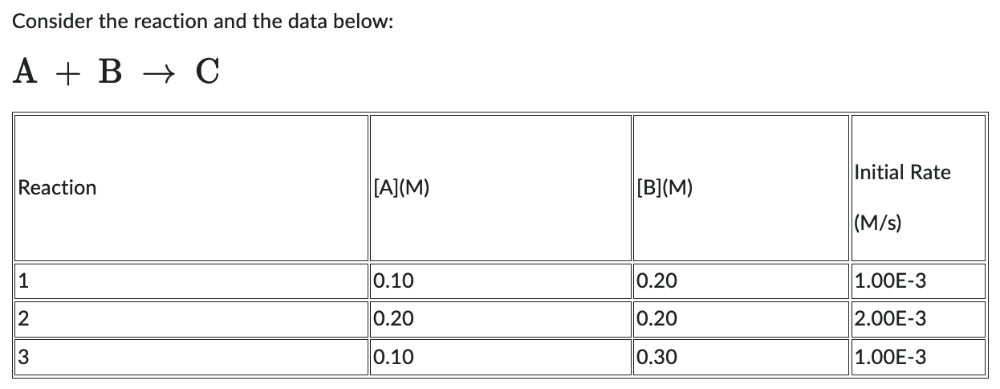
The order with respect to B is:
1 = 1.5
0