viral genetics
...
viruses
- NOT considered living organisms
- NOT cells
- No cell nucleus, organelles, or cytoplasm
- subcellular infectious agents
- rpelicate only inside living host
cells
- are complex nucleoprotein particles
- are obligate intracellular parasites
- a quality that living matter has: autonomous replication (which viruses dont have)
Cellular respiration – a condition for living matter
viruses
- utilize host cell machinery to make components for production of new viral particles
- "a virus is a piece of bad news
wrapped up in a protein"
- bad news comes from the genes they have
the "bad news"
- bad news is the viral nucleic acid (genome)
- = the "brain" of the virus
- -> sufficient information to subordinate host cell's machinery to the viral needs
viral genome -> DNA OR DNA (NEVER BOTH)
viruses
- require getting into living cells to be able to replicate themselves
- can be in
- animals
- plants
- bacteria (bacteriophages)
- genetics of
animal viruses
- -> learn abt structure and function of viral genome and viral proteins
- = better understand and control viral diseases
- = enables us to use viruses as research tools in the lab
viruses
- are organisms w/ greatest genetic diversity in nature subjected
- genome modification processes -> lead to
increased genome variability
- mutation
- recombination
- reassortment
- reassortment adds variability to genomes
- is unique to certain viruses
- natural selection acts on continuously changing viral genomes (because of the 3 processes, mutation recomb, reassortment) = results in great genomic diversity = evolution
- some changes due to recomb, mutation, reassortment are "lethal" -> these viruses will disappear
- some changes provide a better fit/advantage to "survive" for virus-> these changes will be fixed in the genome (will remain/stay)
viral infection
- small number of viruses replicate to produce millions of particles
- ERRORS in copying nucleic acid inevitably occur
- -> lead to MUTATIONS = changes in the nucleic acid'ss tructure of the genome
- many mutations are
LETHAL -> error has profound consequences
- mutated virus unable to replicate (lethal means neutralizes the virus)
non lethal mutations
- eventually eliminated - disappear
- OR
- get fixed in the genome - remain
whether a particular NONLETHAL mutation survives in the genotype, depends on whether the resulting change in the gene product is disadvantageous, neutral, or affords the mutant virus some selective advantage
mutant
changed genetically from wild type
wild type
original strain of a virus from which mutants are selected and to which mutants are compared
original version of a gene
variants could differ by
a single or many mutations
critical for the development of viral genetics and viral research:
??????????? i dont understand this
-
plaque assay for cytolytic viruses
- used to determine viral titer concentration of viruses in a sample
- dev of focus assay was key event in dev of genetic systems for noncytolytic, transforming (tumorigenic) viruses (foci or mini tumors)
- developed by dulbecco early 50's
- first used w/
bacteriophages
- key factor in advances of research in phages and later in animal viruses
A plaque is an area of clearing in a confluent lawn of bacterial growth which represents the spot where a virus has landed, infected the bacteria it encountered, and lysed them
The plaque assay is a well-established method for measuring virus concentration as it relates to infectious dose. The assay relies on determining the number of plaque-forming units (PFU) created in a monolayer of virus-infected cells.
MUTATIONS
- spontaneous
- induced (ex: use mutagens, carcinogens, etc.)
- engineered
3 main changes in viral DNA
spontaneous mutations
- due to mistakes in the normal replication of viral nucleic acid
- accumulate in viral genomes and introduce variations in phenotypes that are subjected to selection pressure during evolution of a virus
- DNA genomes
- mutation rates are LOW (10^-8 to 10^-11) per incorporated nucleotide
- RNA genomes: mutation rate much higher (10^-3 to 10^-4) per
incorporated nucleotide, so 1 out of every 1000 or 10000)
- = low fidelity of RNA genome replication: lack of proofreading activity in RNA replication enzymes
- ex: influenza virus is RNA virus -> new strains continously arise and new vaccines need to be generated every year
- majority of pathogenic viruses are RNA bc they are more variable and aggressive
- RNA genomed viruses mutate much more bc of higher rate of mutation
Induced mutations
- occur when wild type viruses are treated with mutagens or
carcinogens (chemical/physical agents)
-
chemical mutagens classified according to their
mechanism of mutagenesis. Chem substances that include:
- point mutations - single nucleotide altered
- gross chromosomal changes/aberrations - change in number/structure of chromosomes (not in viruses)
- physical mutagens
- ionizing: X and gamma rays = chain break
- nonionizing: UV = induce pyrimidine dimers -> stop any replication possibility bc the structure is altered
-
chemical mutagens classified according to their
mechanism of mutagenesis. Chem substances that include:
basic unit of the DNA molecule: nucleotide
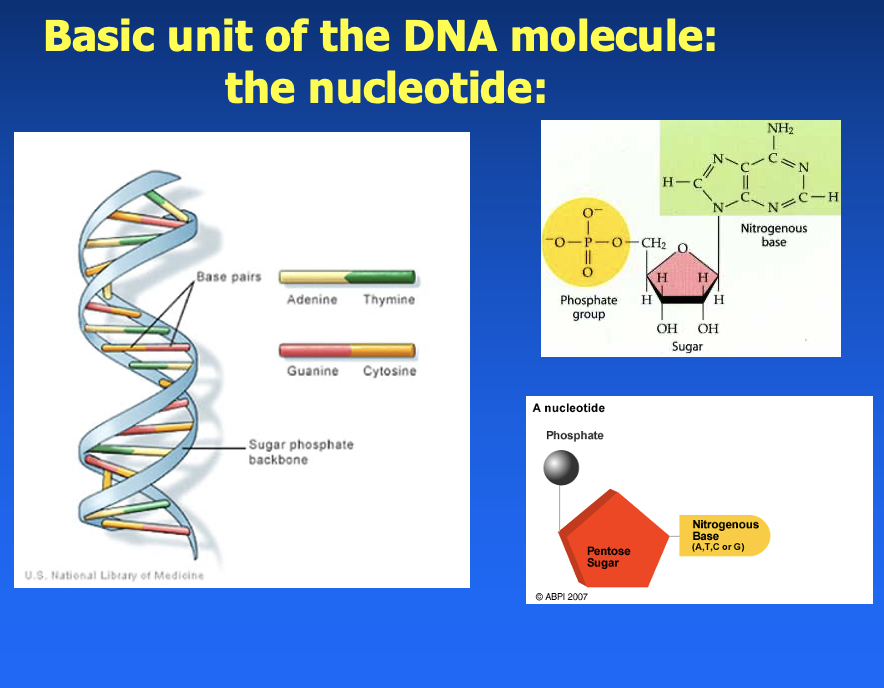
- phosphate
- pentose sugar
- nitrogenous base (A, T, C, G)
point mutations
revert to wild type with certain frequency
-
base pair changes - change by 1 base in nucleotide
- transitions - purine to purine or pyrimidine to pyrimidine
- transversions - purine to pyrimidine or vice versa
- can have diff consequences
- silent - no change in amino acid (codes for same codon), doesnt affect outcome (degeneracy of genetic code)
- missense - change of one aa for another aa
- nonsense - codon termination (UGA, UAA, UGA). Makes codon a stop codon. Problem bc transcription of gene stops = serious mutation
-
frameshift changes - generate a non functional
product
- insertion or deletion of 1 or 2 bases
- cause shift, so codons all change (dont have the same 3 bases in each as before the insertion/deletion)
- codon = 3 bases
engineered mutations
- molecular technique made it possible to introduce nearly any
type of mutation into viruses
- results in expected changes as opposed to results of induced mutagenesis (which are random)
- know the mutations want to produce
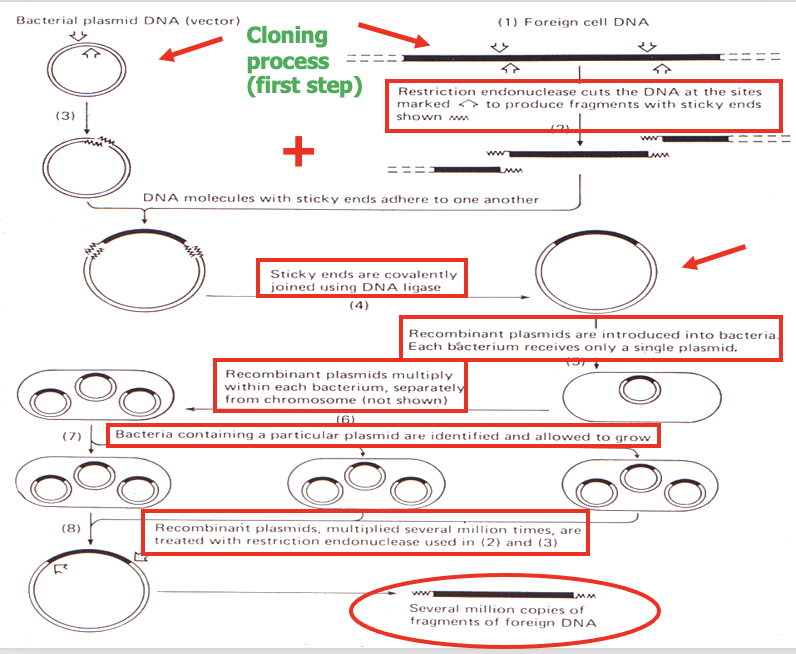
- steps before PCR was invented:
- particular sequence of inetest -> clone it as recombinant DNA molecule
- subject sequence to "in vitro mutagenesis"
- -> put sequence in viral genome
*** GO OVER THIS TO UNDERSTAND IT
cont.
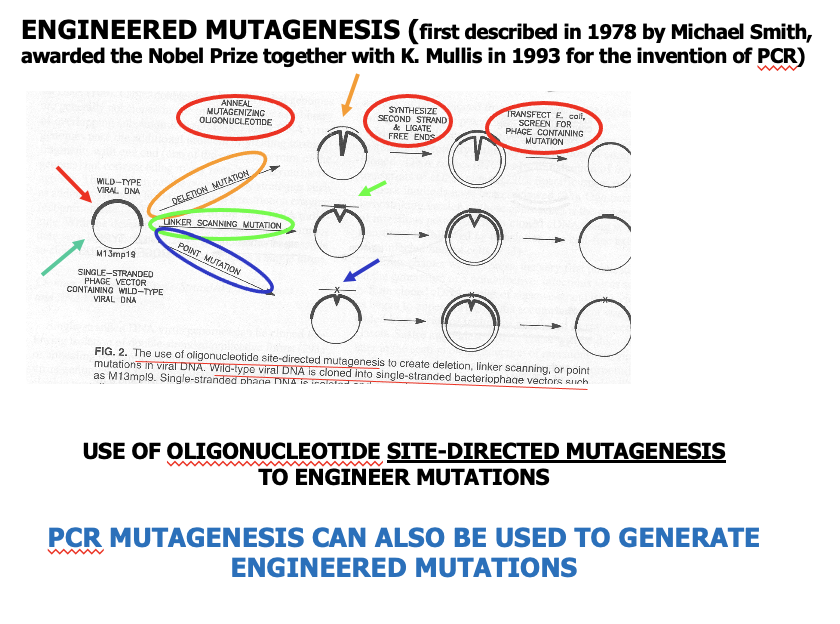
Plasmid w/ region wanna mutate -> anneal the plasmid and sequence
-> engineer it by pairing it w/ sequence and…
Point mutation -> make second strand and transfect/transform… (see slide)
cont?????
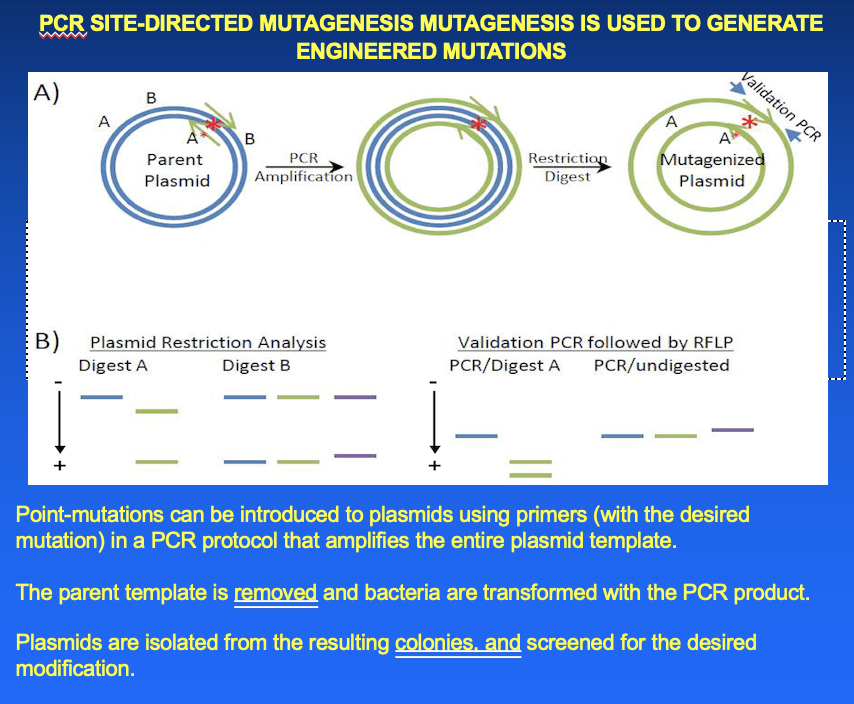
PCR site-directed mutagenesis is used to generate engineered mutations
- point mutations can be introduced to plasmids using primers (with desired mutations) in a PCR protocol that amplifies the entire plasmid template
- the parent template is removed and bacteria are transformed with the PCR product
- plasmids are isolated from the resulting colonies and screened for the desired modification
REVIEW PCR CONCEPT AND STEPS
CRISPR
UPLOAD PIC
clustered regularly interspaced short palindromic repeats
- adapted from naturally occurring genome editing system in bacteria
- bacteria capture snippets of DNA from invading viruses + use them to create DNA segments known as crispr arrays
- crispr arrays allow bacteria to "remember" the viruses (or closely related ones)
- crispr is a technology that can be used to edit genes and will likely change the world
- essence of crispr: a way of finding a specific bit of DNA inside a cell. Next step = alter that piece of DNA
types of mutations according to their phenotypes
genotype is NOT equal to phenotype
- NULL
- TEMPERATURE-SENSITIVE MUTANTS(TS)
- COLD-SENSITIVE MUTATIONS
- PLAGUE MORPHOLOGY MUTATIONS
- HOST RANGE MUTATION
- GENETIC RECOMBINATION BETWEEN VIRUSES
null:
- completely inactivates the function of a gene
- any type of nucleic acid change can yield a null phenotype
- can be due to frameshift, point mutation etc., any type of change)
- not even large deletions will necessary cause the null phenotype: some of these mutations leave intact protein fragments that retain function
- when engineering null mutations, it is safest to delete the entire gene
- null mutant is critical to determine if a gene is essential or not important important for some process
temperature sensitive mutants (ts)
- conditional-lethal phenotypes
- useful in animal viruses
- usually produced by missense mutations that alter nucleotide sequence of a gene -> resulting protein product is unable to maintain functional configuration at the nonpermissive (high) temperature
- protein is able to assume a functional config at a permissive (low) temp = mutant can be propagated
- can theoretically select TS mutations in any viral gene encoding a required gene product
- certain TS mutants are "leaky" - they retain some functional activity at "nonpermissive" temperatures
- bc temp can be
manipulated up and down - these mutants are useful in
temperature shift experiments
- in assessing time during which a gene product required for a certain process
cold sensitive mutations
- conditionally-lethal at low (nonpermissive) temperature and grow nearly as well as wild type at high (permissive) temp
- have many of the time uses as TS mutations and
are explored as attenuation mutations for vaccines
- to attenuate, but not kill the virus, bc it then cannot function or grow at cold temps
plaque morphology mutations
- have altered morphology
- large plaques release virus from the host cell more rapidly than the wild type
- provide useful markers - mutants can illustrate processes like membrane fusion
Center of plaque – original bacteria that died
- Large plaque – virus mutation killed everything rapidly
- Can use as way to assess the number of viruses
host range mutations
- also conditional lethal: selected in a number of viruses: can grow in human but not in hamster cells, or in viral-transformed cells but not in normal cells
- can kill in humans that infect us but not other things
genetic recombination between viruses
- when 2 diff viruses simultaneously infect the same cell
- recombination - exchange in pieces of DNA/RNA btw them
- recombination may occur
- occurs when there is exchange BETWEEN newly synthesized nucleic acid molecules = new nucleic acid molecule
-
2 types recombination
-
reassortment
- in viruses w/ segmented genomes only
- intramolecular recombination
-
reassortment
cont.
UPLOAD PIC
intramolecular recombination
- cut and "paste"
genetic reassortment
- occurs in viruses with segmented genomes
intramolecular recombination
- involves physical exchange of nucleic acid sequences btw viruses during viral replication
- occurs with all dsDNA viruses, presumably bc of strand switching by viral DNA polymerase; it exists also among some RNA viruses
- can also occur btw viral and cellular RNA/DNA
- integration of viral DNA into cellular DNA by recombination occurs in cells transformed by some DNA viruses (adenoviruses)
- in case of retroviruses,
recombination occurs btw DNA provirus and cell DNA. Retroviruses may
pick up a cellular genome to become viral oncogenes
- go in nucleus + can activate things
reassortment
- modality of recombination that occurs btw RNA viruses that have
segmented genomes
- whether they are ssRNA or dsRNA
- in cell infected with 2 of these -> there is an exchange of whole segments/pieces = results in production of various stable reassortment
- occurs in nature + is an important source of genetic variability (ex: flu)
interaction btw viral gene products (proteins)
- protein interactions -> physical recombination or
reassortment btw nucleic acids of diff viruses
- -> permanent heritable change in genomes of recombinant progeny
- totally diff class of interactions:
- no direct association btw parental genomes
- no change in the genomes of the progeny
- interaction btw gene products (proteins)
- complementation
- phenotypic mixing
complementation
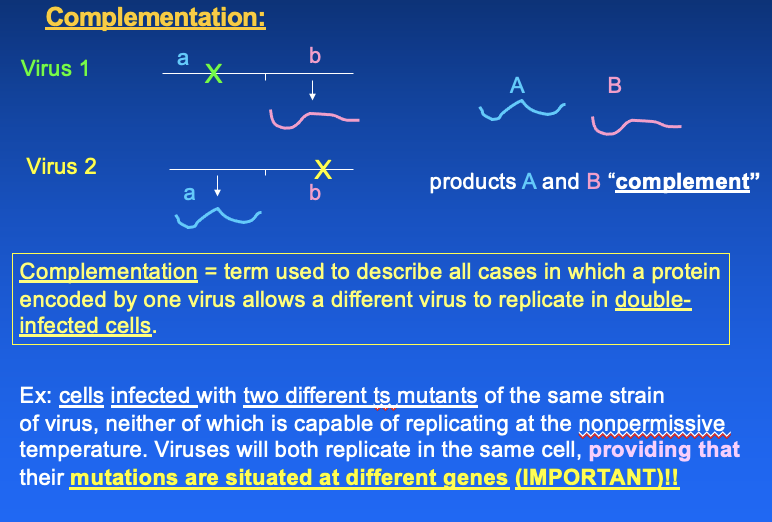
- products of 2 viruses "compliment"
- term used to describe all cases in which a protein encoded by 1 virus allows a diff virus to replicate in double-infected cells
- ex: cells infected w/ 2
diff TS mutants of the same strain of virus, neither is capable of
replicating at the nonpermissive temperature
- BUT both viruses will replicate in the same cell if their mutations are situated at diff genes (IMPORTANT)
Ex: virus 1 have mutation that it cannot make protein a
Virus 2 has mutation on b so cant make the protein. normally they would not be able to grow
- complementation allows the virus to grow well bc they have the proteins they need from each other ( bc they are both in same cell)
Panel of random mutants -> can be allocated to functional groups
functional groups correspond to separate genes
-> complementation mapping
- by testing which mutant comlpements with another
- since no genetic recombination is involved, both parents breed true and the progeny resembles one parent or the other
- complementation can also occur between unrelated viruses
- ex: btw an adenovirus and adeno-associated viruses, or between
SV40 and an adenovirus in monkey cells:
- the first virus serves as a "helper" virus providing a gene product that the second virus requires in prder to be able to replicate in a cell type that is otherwise non permissive for it (Helper virus provides the gene)
phenotype mixing
- proteins from 2 diff viruses are mixed in infected cell
- after mixed infection by 2 viruses that share common features -> some progeny may acquire phenotypic characteristics from both parents (though their genotype remains unchanged)
- ex: coinfection w/ 2 diff viruses)
- envelopes of some of the progeny contain viral antigens derived from each parent
- though each virion contains the nucleic acid of only 1 parent
transcapsidation
transcapsidation- type of phenotypic mixing that occurs when there is a partial or complete exchange of capsids
- 2 diff viruses that replicated will exchange their capsid
see pic
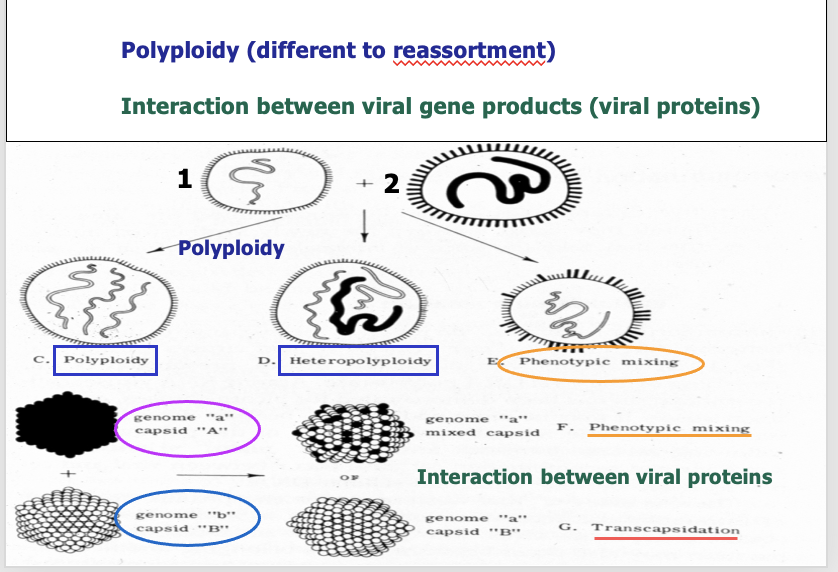
Polyploidy - Included more RNA or DNA than is needed
Hetero – 2 diff viruses. 1 can go into capsid of the other one
Phenotypic mixing –black virus + white virus replicate and interact
- their capsids mix with genome a
- 1 example of mixing of 2 viruses where make hybrid
Transcapsidation
- same 2 viruses replicate in cell -> genome a and capsid B
- another example of mixing 2 viruse s
how are new viruses characterized?
1)Different strains of the suspected new virus would be isolated and distinguished from related viruses from samples of infected subjects.
2)A copy of the genome would be cloned.
3)Efforts to demonstrate biological activity of the cloned DNA or RNA
genome would begin, as would sequence and transcript mapping analyses
4)These analyses would predict potential regulatory elements and viral proteins, and some of their likely functions, such as enzymatic activities.
5) Various kinds of mutations would be engineered in these genetic elements, tested for their effects on viral biology and mapped.
6) The cloned genes could be used to complement viral mutants.
7) The ORFs would be expressed in heterologous systems (E.coli) to help generate antisera in animals to identify the proteins in the infected cells and to identify infected hosts (diagnoses of new cases).
8) The proteins would then be tested for ligand-binding and enzymatic activities and characterized by biochemical and biophysical methods.
Hypothetical chain of events is similar to what took place when HIV was recognized, and more recently, in SARS, COVID-19 and new influenza viruses identification.
Viral genetic important
- discover viruses when arise, etc
Understand concepts
- she wont ask details as much as concepts
Strain of suspected virus isolated from infected ppl
Clone genome