A mutation in the gene coding for a single-polypeptide enzyme results in the substitution of the amino acid serine, which has a polar R group, by the amino acid phenylalanine, which has a nonpolar R group. When researchers test the catalysis of the normal enzyme and the mutated enzyme, they find that the mutated enzyme has much lower activity than the normal enzyme does.
Which of the following most likely explains how the amino acid substitution has resulted in decreased catalytic activity by the mutated enzyme?
A
The substitution decreased the mass of the enzyme so that the mutated enzyme binds more weakly to the substrate than the normal enzyme does.
B
The substitution altered the secondary and tertiary structure of the enzyme so that the mutated enzyme folds into a different shape than the normal enzyme does.
C
The substitution caused many copies of the mutated enzyme to cluster together and compete for substrate to bind.
D
The substitution caused the directionality of the enzyme to change such that the amino terminus of the normal enzyme has become the carboxy terminus of the mutated enzyme.
B. The substitution altered the secondary and tertiary structure of the enzyme so that the mutated enzyme folds into a different shape than the normal enzyme does.
Bacteriophages are viruses that infect bacteria. In an experiment, bacteriophages were labeled with either radioactive phosphorus or radioactive sulfur. The labeled bacteriophages were incubated with bacteria for a brief amount of time and then removed. The infected bacteria cells were found to contain significant amounts of radioactive phosphorus but not radioactive sulfur.
Based on the results of the experiment, which of the following types of molecules did the bacteriophages most likely inject into the bacteria cells?
Responses
A
Simple carbohydrate
B
Amino acid
C
DNA
D
Polypeptide
C
DNA
Which of the following is responsible for the cohesive property of water?
Responses
A
Hydrogen bonds between the oxygen atoms of two adjacent water molecules
B
Covalent bonds between the hydrogen atoms of two adjacent water molecules
C
Hydrogen bonds between the oxygen atom of one water molecule and a hydrogen atom of another water molecule
D
Covalent bonds between the oxygen atom of one water molecule and a hydrogen atom of another water molecule
E
Hydrogen bonds between water molecules and other types of molecules
C. Hydrogen bonds between the oxygen atom of one water molecule and a hydrogen atom of another water molecule
A typical bag of fertilizer contains high levels of nitrogen, phosphorus, and potassium but trace amounts of magnesium and calcium. Which of the following best matches the fertilizer component with the molecule in which it will be incorporated by organisms in the area?
Responses
A
Nitrogen will be incorporated into nucleic acids.
B
Phosphorus will be incorporated into amino acids.
C
Potassium will be incorporated into lipids.
D
Magnesium will be incorporated into carbohydrates.
A
Nitrogen will be incorporated into nucleic acids.
If 30% of the nucleotides in a single-stranded RNA molecule are adenine, then what percent are expected to be thymine?
Responses
A
0%
B
20%
C
30%
D
70%
A
0%
Amylase is an enzyme that converts carbohydrate polymers into monomers. Glycogen synthase is one of the enzymes involved in converting carbohydrate monomers into polymers.
Which of the following best explains the reactions of these enzymes?
Responses
A
Amylase aids in the removal of a water molecule to break covalent bonds whereas glycogen synthase aids in the addition of a water molecule to form covalent bonds.
B
Amylase aids in the addition of a water molecule to break covalent bonds whereas glycogen synthase aids in the removal of a water molecule to form covalent bonds.
C
Amylase aids in the addition of a water molecule to form covalent bonds whereas glycogen synthase aids in the removal of a water molecule to break covalent bonds.
D
Amylase aids in the removal of a water molecule to form covalent bonds whereas glycogen synthase aids in the addition of a water molecule to break covalent bonds.
B
Amylase aids in the addition of a water molecule to break covalent bonds whereas glycogen synthase aids in the removal of a water molecule to form covalent bonds.
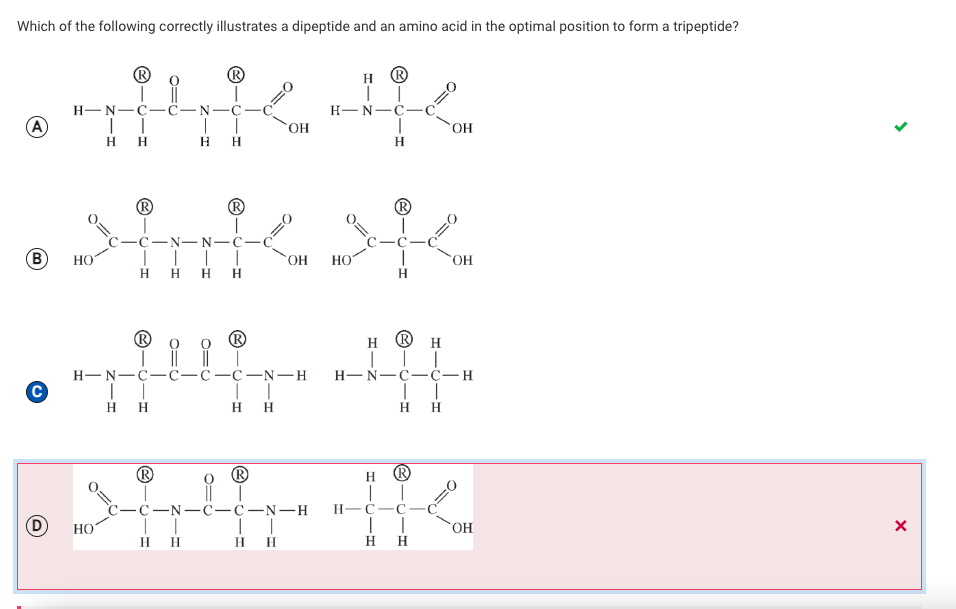
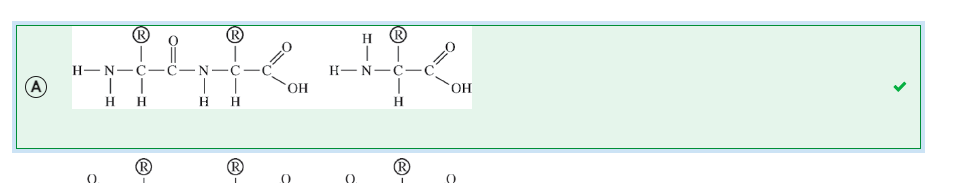
A researcher measured the temperature at which two different samples of double-stranded DNA denature (separate into single strands). Sample 1 denatured at a significantly lower temperature than sample 2 did. Based on the data, the researcher claims that the DNA in sample 2 is composed of a higher percentage of guanine and cytosine than the DNA in sample 1 is.
Which of the following best supports the researcher’s claim?
A
The bonds between guanine and cytosine are covalent bonds, which require more energy to disrupt than those between adenine and thymine.
B
Guanine-cytosine pairs denature at a higher temperature because they have more hydrogen bonds between them than adenine-thymine pairs do.
C
Adenine-thymine pairs require less energy to separate because adenine and thymine are both single-ring bases.
D
Guanine-cytosine pairs require more energy to separate because one is a purine and one is a pyrimidine.
B
Guanine-cytosine pairs denature at a higher temperature because they have more hydrogen bonds between them than adenine-thymine pairs do.
Which of the following best explains how higher concentrations of nitrogen and phosphorus contribute to eutrophication?
Responses'
A
An increase in the population of algae results in more nitrogen and phosphorus in the water, causing severe eutrophication.
B
Both bacteria and algae require nitrogen and phosphorus, so the algae must grow faster to compete with bacteria.
C
Nitrogen and phosphorus stimulate oxidative phosphorylation, which consumes the available oxygen in the water.
D
Algae require nitrogen and phosphorus to build macromolecules, so higher concentrations of these nutrients can result in algal blooms.
D
Algae require nitrogen and phosphorus to build macromolecules, so higher concentrations of these nutrients can result in algal blooms.
Used to carry the genetic code
Responses
A
Proteins
B
Carbohydrates
C
Nucleic acids
D
Lipids
E
Steroids
C
Nucleic acids
Which of the following best describes the hydrolysis of carbohydrates?
Responses
A
The removal of a water molecule breaks a covalent bond between sugar monomers.
B
The removal of a water molecule forms a covalent bond between sugar monomers.
C
The addition of a water molecule breaks a covalent bond between sugar monomers.
D
The addition of a water molecule forms a covalent bond between sugar monomers.
C
The addition of a water molecule breaks a covalent bond between sugar monomers.
A common test for liver function involves sprinkling sulfur powder onto a sample of urine (mostly water with dissolved bodily waste). Sulfur powder sprinkled on a sample from an individual with impaired liver function will sink because the urine contains a high level of bile salts, while the sulfur powder sprinkled on normal urine samples will float.
Which of the following best explains why bile salts cause the sulfur powder to sink?
A
Bile salts decrease the surface tension of the urine sample.
B
Bile salts increase the water potential of the urine.
C
Bile salts increase the density of the urine sample.
D
Bile salts decrease the strength of the covalent bonds within a water molecule.
A
Bile salts decrease the surface tension of the urine sample.
A feature of organic compounds NOT found in inorganic compounds is the presence of
Responses
A
ionizing chemical groups
B
electrons
C
carbon atoms covalently bonded to each other
D
oxygen
E
hydrogen bonds
C
carbon atoms covalently bonded to each other
Which of the following best explains why a cell’s plasma membrane is composed of two layers of phospholipids rather than just a single layer?
Responses
A
Having two oppositely oriented layers of phospholipids allows only the hydrophilic heads to interact with water inside and outside of the cell.
B
Having two oppositely oriented layers of phospholipids allows the hydrophilic heads to repel water both inside and outside of the cells.
C
Having two identically oriented layers of phospholipids gives cells more protection from the exterior environment than just a single layer would.
D
Having two identically oriented layers of phospholipids allows for the production of vacuoles while still maintaining a protective barrier.
Having two oppositely oriented layers of phospholipids allows only the hydrophilic heads to interact with water inside and outside of the cell.
The amino acid in Figure 1 is found in a region of a polypeptide that folds away from water. Which part of the amino acid most likely contributes to the hydrophobic behavior of this region of the polypeptide?
ResponsesA
Amine (NH2) group
B
Carboxyl (COOH) group
C
Methyl (CH3) group
D
Hydrogen (H) atom
C
Methyl (CH3) group
Which of the following best describes the process by which gas from the atmosphere is obtained by plants and used to build lipids?
Responses
A
Gas is fixed by plants as part of the sulfur cycle.
B
Gas is fixed by plants as part of the nitrogen cycle.
C
Gas is directly obtained by plants as part of the carbon cycle.
D
Gas is directly obtained by plants as part of the magnesium cycle.
C
Gas is directly obtained by plants as part of the carbon cycle.
The diagram shows how water can adhere to the xylem in the stems of plants, which contributes to water movement in the plant. Which of the following best explains how water is able to move upward from the roots of a plant, through its xylem in the stem, and out to the leaves?
A
Water is polar, and the walls of the xylem are nonpolar. Water molecules have the ability to form hydrogen bonds with one another but not with the xylem walls.
B
Water is nonpolar, and the walls of the xylem are polar. Water molecules are able to form hydrogen bonds with the xylem walls, and they are pulled up the xylem.
C
Water and the xylem are both nonpolar. Water molecules have the ability to form hydrogen bonds with one another but not with the xylem walls.
D
Water and the xylem are both polar. Water molecules have the ability to form hydrogen bonds with each other and with the walls of the xylem.
D
Water and the xylem are both polar. Water molecules have the ability to form hydrogen bonds with each other and with the walls of the xylem.
Humans produce sweat as a cooling mechanism to maintain a stable internal temperature. Which of the following best explains how the properties of water contribute to this physiological process?
Responses
A
The high specific heat capacity of water allows the body to absorb a large amount of excess heat energy.
B
The high heat of vaporization of water allows the body to remove excess heat through a phase change of water from liquid to gas.
C
The high surface tension of water contributes to the physical process by which water leaves the body.
D
The high melting temperature of water allows the body to remove excess heat through a phase change of water from solid to liquid.
B
The high heat of vaporization of water allows the body to remove excess heat through a phase change of water from liquid to gas.
The synthesis of protein or carbohydrate polymers always produces which of the following as a byproduct?
Responses
A
ATP
B
Oxygen
C
Carbon dioxide
D
Urea
E
Water
E
Water
The sequences for two short fragments of DNA are shown above. Which of the following is one way in which these two segments would differ?
Responses
A
Segment 1 would not code for mRNA because both strands have T, a base not found in RNA.
B
Segment 1 would be more soluble in water than segment 2 because it has more phosphate groups.
C
Segment 1 would become denatured at a lower temperature than would segment 2 because A-T base pairs have two hydrogen bonds whereas G-C base pairs have three.
D
Segment 1 must be from a prokaryote because it has predominantly A-T base pairs.
C
Segment 1 would become denatured at a lower temperature than would segment 2 because A-T base pairs have two hydrogen bonds whereas G-C base pairs have three.
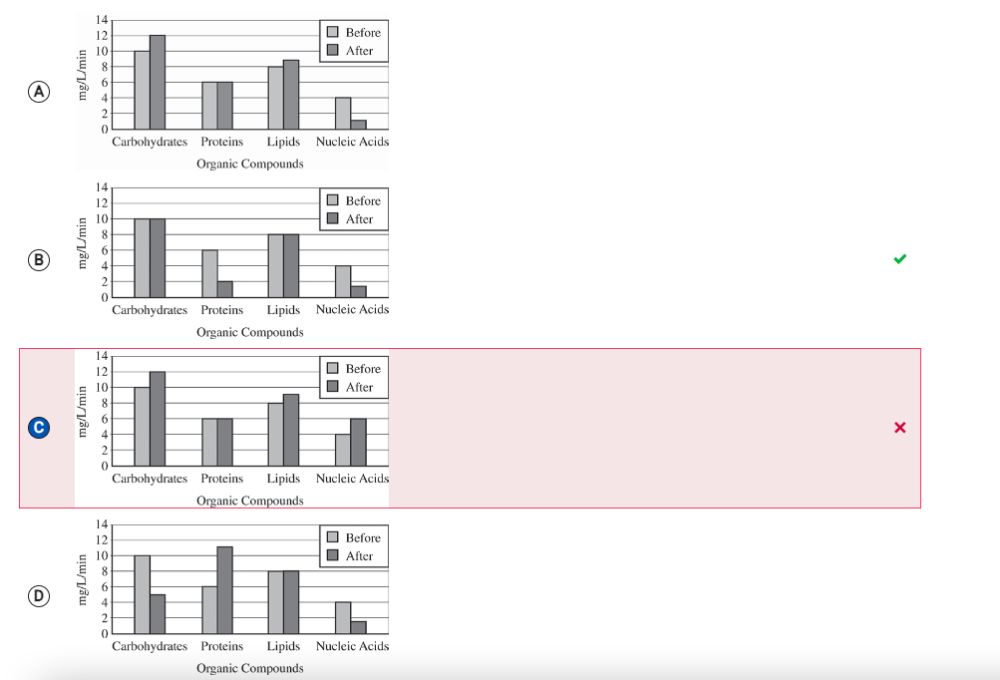
A culture of Spirogyra (an autotrophic alga) is maintained in a water solution containing dissolved carbon dioxide and a source of phosphates but lacking nitrogen compounds. A researcher determines the rates of synthesis of several organic compounds found in the Spirogyra before and after several weeks in the water solution. Which of the following graphs best illustrates a likely result of the experiment?
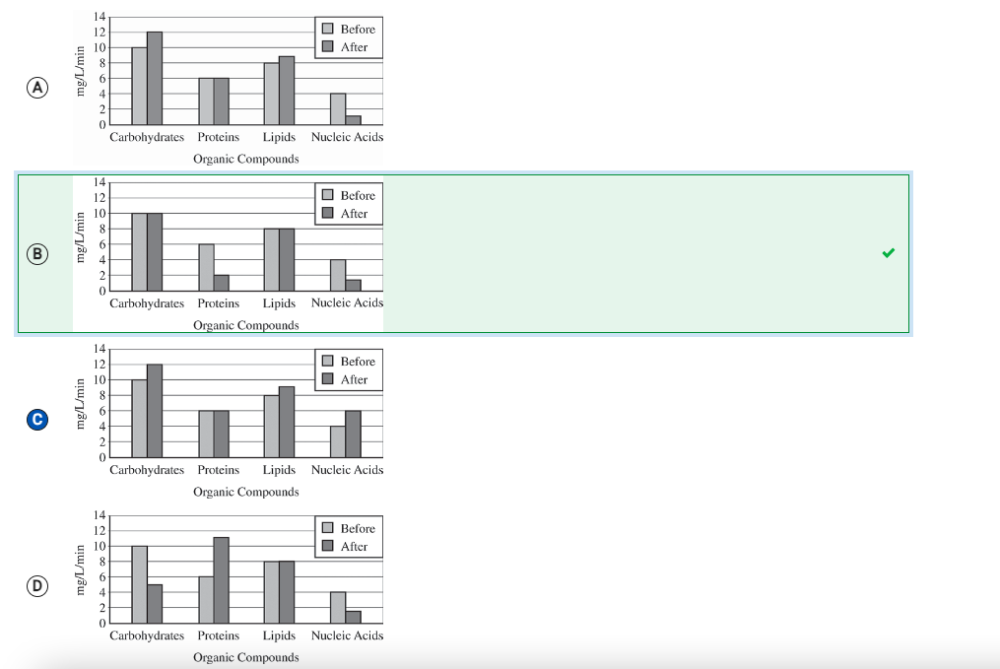
B
Ultraviolet (UV) radiation can damage DNA by breaking weak bonds. Which of the following best explains how this occurs?
Responses
A
UV radiation disrupts the double helix structure by breaking the covalent bonds between the nitrogenous base pairs.
B
UV radiation disrupts the double helix structure by breaking the hydrogen bonds between the nitrogenous base pairs.
C
UV radiation is able to break DNA strands in two by breaking covalent bonds between the sugar-phosphate backbone molecules.
D
UV radiation is able to break DNA strands in two by breaking hydrogen bonds between the sugar-phosphate backbone molecules.
B
UV radiation disrupts the double helix structure by breaking the hydrogen bonds between the nitrogenous base pairs.
A student analyzed a viral genome and found that the genome had the following nucleotide composition.
• 28% adenine
• 20% thymine
• 35% cytosine
• 17% guanine
Which of the following best describes the structure of the viral genome?
A
Double-stranded DNA
B
Single-stranded DNA
C
Double-stranded RNA
D
Single-stranded RNA
B
Single-stranded DNA
Which of the following is most directly responsible for water’s unique properties?
Responses
A
It contains oxygen atoms.
B
It contains hydrogen atoms.
C
It is an ionic compound.
D
It forms hydrogen bonds.
E
It is nonpolar.
D
It forms hydrogen bonds.
Based on Figure 1, the amino acids in region A are most likely to have which of the following characteristics?
Responses
A
Most amino acids will be hydrophobic because they interact most favorably with the phospholipid tails.
B
Most amino acids will be hydrophilic because they interact most favorably with the phospholipid heads.
C
Most amino acids will be ionic amino acids because they interact most favorably with the phospholipid tails.
D
Most amino acids will be polar amino acids because they interact most favorably with the phospholipid heads.
A
Most amino acids will be hydrophobic because they interact most favorably with the phospholipid tails.
A researcher analyzed four different samples of macromolecules, where all macromolecules in each sample are of the same type. The researcher measured the percent of carbon, oxygen, hydrogen, nitrogen, phosphorus, and sulfur atoms in each sample. The results are shown in Table 1.
Which of the following claims is best supported by the data in Table 1 ?
Responses
A
Sample A contains nucleic acids.
B
Sample B contains protein.
C
Sample C contains nucleic acids.
D
Sample D contains protein.
B
Sample B contains protein.
Scientists examined the folded structure of a purified protein resuspended in water and found that amino acids with nonpolar R groups were primarily buried in the middle of the protein, whereas amino acids with polar R groups were primarily on the surface of the protein. Which of the following best explains the location of the amino acids in the folded protein?
Responses
A
Polar R groups on the surface of the protein can form ionic bonds with the charged ends of the water molecules.
B
Polar R groups are too bulky to fit in the middle of the protein and are pushed toward the protein’s surface.
C
Nonpolar R groups that cannot form hydrogen bonds with water are pushed into the middle of the protein.
D
Nonpolar R groups from different parts of the protein form covalent bonds with each other to maintain the protein’s structure.
C
Nonpolar R groups that cannot form hydrogen bonds with water are pushed into the middle of the protein.
A chemical binds to a protein composed of a single polypeptide chain and prevents the formation of an alpha helix that is typically formed in the absence of the chemical. Which of the following best describes the effect the chemical has on the structure of the protein?
Responses
A
The primary structure held together by covalent bonds is affected.
B
The secondary structure held together by hydrogen bonds is affected.
C
The secondary, tertiary, and quaternary structures are affected.
D
All levels of protein structure are affected.
C
The secondary, tertiary, and quaternary structures are affected.
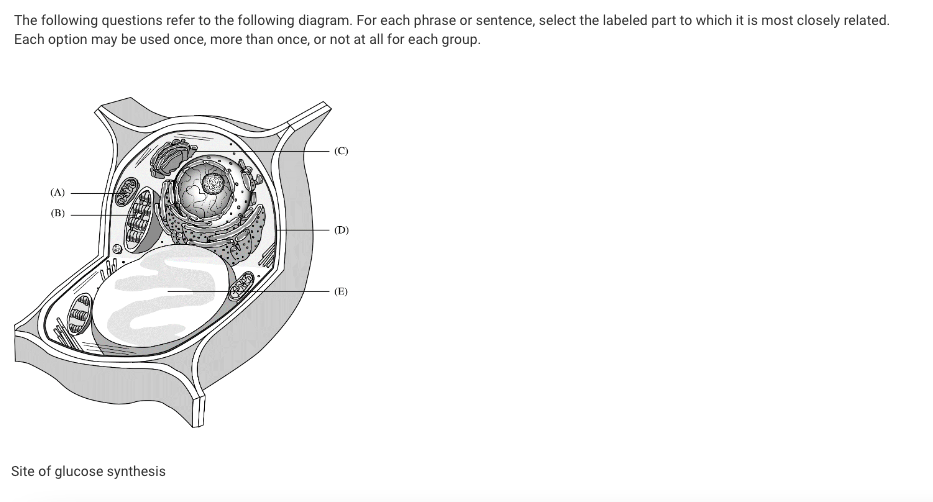
Site of glucose synthesis
A
B
C
D
B
Site of conversion of chemical energy of glucose to ATP
A
B
C
D
E
A
Site of modification and packaging of proteins and lipids prior to export from the cell
Responses
A
B
C
D
E
C
Site of transport of materials into and out of the cell
Responses
A
B
C
D
E
D
Evolved from a photoautotrophic prokaryote
Responses
A
B
C
D
E
B
Liver cells manufacture glycoproteins, while adipose cells store fat. Which of the following subcellular structures is likely to be more prominent in liver cells than in adipose cells?
Responses
A
Nucleus
B
Golgi apparatus
C
Cytoskeleton
D
Plasma membrane
B
Golgi apparatus
Which of the following best describes the hydrolysis of carbohydrates?
Responses
A
The removal of a water molecule breaks a covalent bond between sugar monomers.
B
The removal of a water molecule forms a covalent bond between sugar monomers.
C
The addition of a water molecule breaks a covalent bond between sugar monomers.
D
The addition of a water molecule forms a covalent bond between sugar monomers.
C
The addition of a water molecule breaks a covalent bond between sugar monomers.
The figure above represents a rough endoplasmic reticulum. Which of the following best describes the role of the structure labeled Y?
Responses
A
Structure Y contributes the raw materials required for the synthesis of proteins.
B
Structure Y packages proteins for export from the cell.
C
Structure Y is the location where proteins are synthesized.
D
Structure Y contains enzymes that cut and activate proteins.
C
Structure Y is the location where proteins are synthesized.

Simple cuboidal epithelial cells line the ducts of certain human exocrine glands. Various materials are transported into or out of the cells by diffusion. (The formula for the surface area of a cube is 6 X S 2, and the formula for the volume of a cube is S 3, where S = the length of a side of the cube.)
Which of the following cube-shaped cells would be most efficient in removing waste by diffusion?
A
B
C
D
A
Ultraviolet (UV) radiation can damage DNA by breaking weak bonds. Which of the following best explains how this occurs?
Responses
A
UV radiation disrupts the double helix structure by breaking the covalent bonds between the nitrogenous base pairs.
B
UV radiation disrupts the double helix structure by breaking the hydrogen bonds between the nitrogenous base pairs.
C
UV radiation is able to break DNA strands in two by breaking covalent bonds between the sugar-phosphate backbone molecules.
D
UV radiation is able to break DNA strands in two by breaking hydrogen bonds between the sugar-phosphate backbone molecules.
B
UV radiation disrupts the double helix structure by breaking the hydrogen bonds between the nitrogenous base pairs.
Which of the following describes the most likely location of cholesterol in an animal cell?
Responses
A
Embedded in the plasma membrane
B
Dissolved in the cytosol
C
Suspended in the stroma of the chloroplast
D
Bound to free ribosomes
A
Embedded in the plasma membrane
The diagram above represents a typical rod-shaped bacterium. Which of the following best describes a feature shown in the diagram that is unique to archaea and bacteria?
Responses
A
The organism is surrounded by a cell wall.
B
The organism contains ribosomes.
C
The organism does not have a nuclear membrane surrounding its genetic material.
D
The organism is not capable of making or providing itself with ATP.
C
The organism does not have a nuclear membrane surrounding its genetic material.
In an experiment, the efficiency of oxygen exchange across the plasma membrane is being assessed in four artificial red blood cells. The table above lists some properties of those artificial cells. Other conditions being equal, which artificial cell is predicted to be the most efficient in exchanging oxygen with the environment by diffusion?
Responses
A
The cuboidal cell
B
The tetrahedral cell
C
The cylindrical cell
D
The spherical cell
A
The cuboidal cell
Humans produce sweat as a cooling mechanism to maintain a stable internal temperature. Which of the following best explains how the properties of water contribute to this physiological process?
Responses
A
The high specific heat capacity of water allows the body to absorb a large amount of excess heat energy.
B
The high heat of vaporization of water allows the body to remove excess heat through a phase change of water from liquid to gas.
C
The high surface tension of water contributes to the physical process by which water leaves the body.
D
The high melting temperature of water allows the body to remove excess heat through a phase change of water from solid to liquid.
B
The high heat of vaporization of water allows the body to remove excess heat through a phase change of water from liquid to gas.
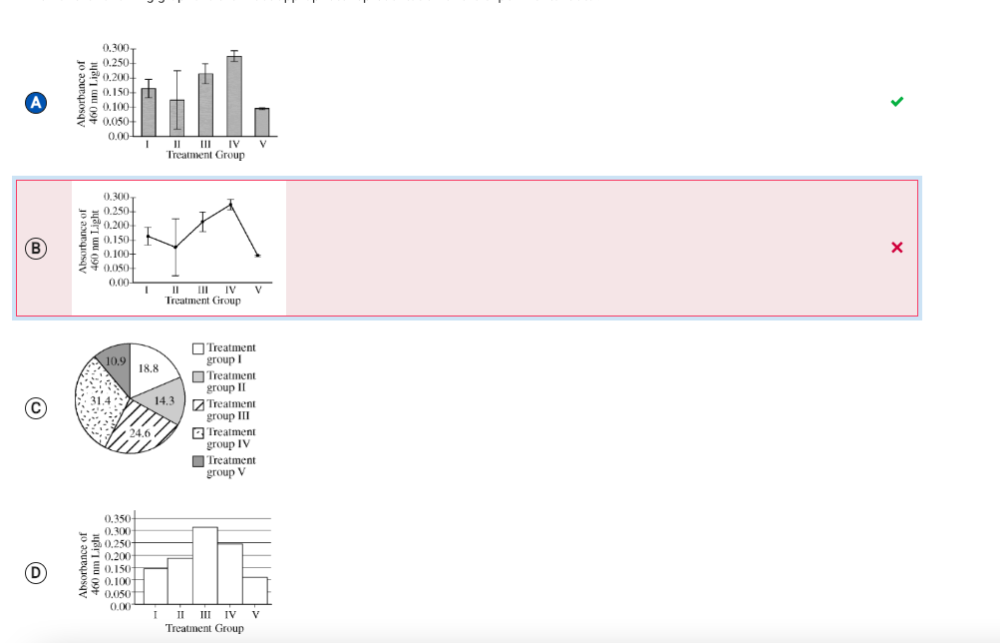
A student formulated a hypothesis that water-soluble pollutants damage living organisms by increasing the permeability of cellular membranes. To test the hypothesis, the student investigated the effect of isopropanol and acetone on beet root cells. The vacuoles of beet root cells contain large amounts of betacyanin, a water-soluble pigment that is released into the extracellular environment as a result of increased membrane permeability.
The student prepared identical samples of beet root tissue and incubated each sample for 15 minutes in the specific solution for that group. At the end of the incubation period, the student measured the absorbance of 460 nm light for each sample. A greater concentration of betacyanin in the solution surrounding the beet root cells results in a greater absorbance of 460 nm light. The results of the experiment are shown in the table above.
Which of the following graphs is the most appropriate representation of the experimental data?
Responses
A
B
C
D
A
A student formulated a hypothesis that water-soluble pollutants damage living organisms by increasing the permeability of cellular membranes. To test the hypothesis, the student investigated the effect of isopropanol and acetone on beet root cells. The vacuoles of beet root cells contain large amounts of betacyanin, a water-soluble pigment that is released into the extracellular environment as a result of increased membrane permeability.
The student prepared identical samples of beet root tissue and incubated each sample for 15 minutes in the specific solution for that group. At the end of the incubation period, the student measured the absorbance of 460 nm light for each sample. A greater concentration of betacyanin in the solution surrounding the beet root cells results in a greater absorbance of 460 nm light. The results of the experiment are shown in the table above.
Which of the following is the dependent variable in the experiment?
Responses
A
The percent by volume of isopropanol in the treatment solutions
B
The percent by volume of water in the treatment solutions
C
The length of time each sample was incubated in the treatment solutions
D
The absorbance of 460 nm light by the treatment solutions
D
The absorbance of 460 nm light by the treatment solutions
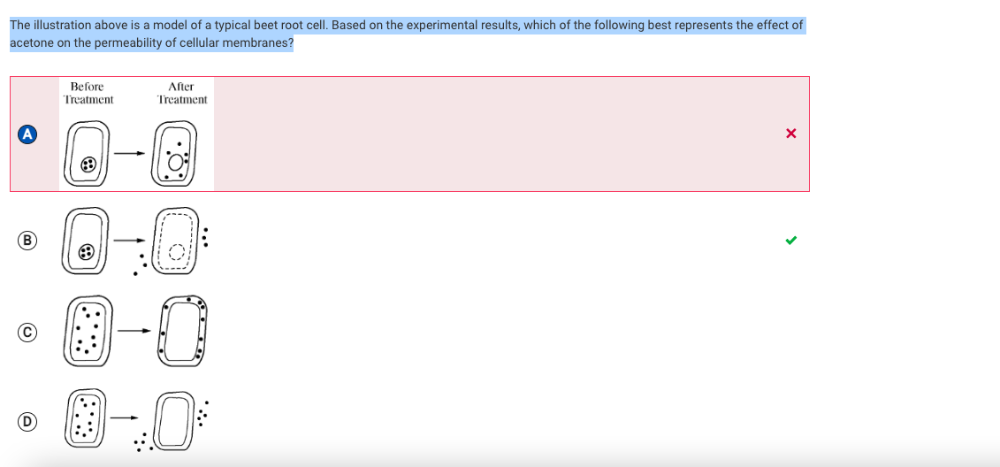
The illustration above is a model of a typical beet root cell. Based on the experimental results, which of the following best represents the effect of acetone on the permeability of cellular membranes?
A
B
C
D
B
A student formulated a hypothesis that water-soluble pollutants damage living organisms by increasing the permeability of cellular membranes. To test the hypothesis, the student investigated the effect of isopropanol and acetone on beet root cells. The vacuoles of beet root cells contain large amounts of betacyanin, a water-soluble pigment that is released into the extracellular environment as a result of increased membrane permeability.
The student prepared identical samples of beet root tissue and incubated each sample for 15 minutes in the specific solution for that group. At the end of the incubation period, the student measured the absorbance of 460 nm light for each sample. A greater concentration of betacyanin in the solution surrounding the beet root cells results in a greater absorbance of 460 nm light. The results of the experiment are shown in the table above.
The student analyzed the data from the investigation and concluded that the estimate of the mean of one treatment group was unreliable. Which of the following identifies the treatment group most likely to have provided an unreliable estimate of the mean, and correctly explains why the estimate appears unreliable?
Responses
A
Treatment group II; it has a lower than expected mean absorbance and the largest standard error of the mean.
B
Treatment group III; it has a higher than expected mean absorbance and the largest standard error of the mean.
C
Treatment group IV; it has a higher than expected mean absorbance and the smallest standard error of the mean.
D
Treatment group V; it has a lower than expected mean absorbance and the smallest standard error of the mean.
A
Treatment group II; it has a lower than expected mean absorbance and the largest standard error of the mean.
Paramecia are unicellular protists that have contractile vacuoles to remove excess intracellular water. In an experimental investigation, paramecia were placed in salt solutions of increasing osmolarity. The rate at which the contractile vacuole contracted to pump out excess water was determined and plotted against osmolarity of the solutions, as shown in the graph. Which of the following is the correct explanation for the data?
Responses
A
At higher osmolarity, lower rates of contraction are required because more salt diffuses into the paramecia.
B
The contraction rate increases as the osmolarity decreases because the amount of water entering the paramecia by osmosis increases.
C
The contractile vacuole is less efficient in solutions of high osmolarity because of the reduced amount of ATP produced from cellular respiration.
D
In an isosmotic salt solution, there is no diffusion of water into or out of the paramecia, so the contraction rate is zero.
B
The contraction rate increases as the osmolarity decreases because the amount of water entering the paramecia by osmosis increases.
Which of the following processes is most likely to occur as a result of an animal cell receiving a signal to initiate apoptosis?
Responses
A
Ribosomes will translate mRNA to produce proteins.
B
Vesicles will release extracellular growth factors via exocytosis.
C
Lysosomes will release digestive enzymes into the cytosol.
D
Vacuoles will fuse with the cellular membrane.
C
Lysosomes will release digestive enzymes into the cytosol.
A human kidney filters about 200 liters of blood each day. Approximately two liters of liquid and nutrient waste are excreted as urine. The remaining fluid and dissolved substances are reabsorbed and continue to circulate throughout the body. Antidiuretic hormone (ADH) is secreted in response to reduced plasma volume. ADH targets the collecting ducts in the kidney, stimulating the insertion of aquaporins into their plasma membranes and an increased reabsorption of water.
If ADH secretion is inhibited, which of the following would initially result?
Responses
A
The number of aquaporins would increase in response to the inhibition of ADH.
B
The person would decrease oral water intake to compensate for the inhibition of ADH.
C
Blood filtration would increase to compensate for the lack of aquaporins.
D
The person would produce greater amounts of dilute urine.
D
The person would produce greater amounts of dilute urine.
The manner in which several different ions and molecules move through a cell membrane is shown in the diagram above. For each ion or molecule, the relative concentration on each side of the membrane is indicated. Which of the following accurately describes one of the movements taking place?
Responses
A
Glucose is transported into the cell by active transport.
B
Na+ is transported into the cell by active transport.
C
The movement of glucose through the membrane requires ATP hydrolysis.
D
Na+ transport out of the cell requires ATP hydrolysis.
D
Na+ transport out of the cell requires ATP hydrolysis.
Membrane-bound organelles have been an important component in the evolution of complex, multicellular organisms. Which of the following best summarizes an advantage of eukaryotic cells having internal membranes?
Responses
A
Eukaryotic cells are able to reproduce faster because of the presence of organelles.
B
Some organelles, such as mitochondria and chloroplasts, are similar to prokaryotic cells in structure.
C
Organelles isolate specific reactions, increasing metabolic efficiency.
D
Compartmentalization leads to a higher mutation rate in DNA, which leads to more new species.
C
Organelles isolate specific reactions, increasing metabolic efficiency.
Based on Table 1, which of the following best explains the difference in water potential between certain solutions and the grapes?
Responses
A
NaCl and tap water have a lower water potential because these two solutions caused the grape to gain water.
B
Grape soda and NaCl have a lower water potential because these two solutions caused the grape to lose water.
C
Tap water and grape juice have a lower water potential because these two solutions caused the grape to lose water.
D
Grape soda and grape juice have a lower water potential because these two solutions caused the grape to gain water.
B
Grape soda and NaCl have a lower water potential because these two solutions caused the grape to lose water.
Based on Table 1, which of the following percentages is closest to the solute concentration of the grape?
Responses
A
0.0%
B
1.3%
C
5.5%
D
10.1%
C
5.5%
Based on the data in Table 1, which of the following best evaluates the student’s hypothesis?
Responses
A
The hypothesis is supported because the mass of the grape decreased in the grape juice.
B
The hypothesis is supported because the grape juice has a greater solute potential than the grape has.
C
The hypothesis is not supported because the grape was isotonic to the grape juice.
D
The hypothesis is not supported because the mass of the grape increased in the grape juice.
D
The hypothesis is not supported because the mass of the grape increased in the grape juice.
Assuming a negligible pressure potential, which of the following best predicts the net movement of the small diffusible solutes and water in the second experiment (Table 2) ?
Responses
A
Small diffusible solutes will diffuse into the grape cells, followed by water.
B
Small diffusible solutes will diffuse out of the grape cells and water will diffuse into the cells.
C
Small diffusible solutes will diffuse out of the grape cells, followed by water.
D
Small diffusible solutes will diffuse into the grape cells and water will diffuse out of the cells.
A
Small diffusible solutes will diffuse into the grape cells, followed by water.
Mercurial sulfhydryl is an inhibitor of aquaporins. Which of the following is the most likely effect of adding mercurial sulfhydryl to the distilled water solution?
Responses
A
The grape cells will burst because of excess water entering by active transport.
B
The grape cells will gain more water because of the activation of the transport protein.
C
The grape cells will shrink because active transport has been inhibited.
D
The grape cells will gain water more slowly because of a lack of facilitated diffusion.
D
The grape cells will gain water more slowly because of a lack of facilitated diffusion.
Which of the following best explains why larger grapes have a different rate of water absorption per gram of mass than smaller grapes do?
Responses
A
The rate is slower because smaller grapes have a larger surface-area-to-volume ratio than the larger grapes do.
B
The rate is slower because larger grapes have a larger surface-area-to-volume ratio than the smaller grapes do.
C
The rate is slower because smaller grapes can expand more than larger grapes to hold excess water.
D
The rate is slower because larger grapes have more volume to hold excess water than smaller grapes do.
A
The rate is slower because smaller grapes have a larger surface-area-to-volume ratio than the larger grapes do.
Which of the following best predicts which diagrammed microscope view the laboratory worker would see and best explains why?
Responses
A
View 1 because RBC membranes are freely permeable to water
B
View 2 because the RBCs use energy to allow sodium entry and to pump water out
C
View 2 because the rate of water movement into the RBCs equals the rate of water movement out of the cells
D
View 3 because the sodium-potassium pumps in the RBC membranes use energy to keep the sodium out but allow water to freely flow into the cells
C
View 2 because the rate of water movement into the RBCs equals the rate of water movement out of the cells
Which of the following groups of cellular components are found in eukaryotic cells but not prokaryotic cells?
Responses
A
Ribosomes, a nucleus, and chloroplasts
B
Circular chromosomes, mitochondria, and an endoplasmic reticulum
C
A nucleus, ribosomes, and cell walls
D
An endoplasmic reticulum, mitochondria, and a nucleus
D
An endoplasmic reticulum, mitochondria, and a nucleus
Bacteriophages are viruses that infect bacteria. In an experiment, bacteriophages were labeled with either radioactive phosphorus or radioactive sulfur. The labeled bacteriophages were incubated with bacteria for a brief amount of time and then removed. The infected bacteria cells were found to contain significant amounts of radioactive phosphorus but not radioactive sulfur.
Based on the results of the experiment, which of the following types of molecules did the bacteriophages most likely inject into the bacteria cells?
Responses
A
Simple carbohydrate
B
Amino acid
C
DNA
D
Polypeptide
C
DNA
A scientist is developing a mathematical model of cells of different shapes. To construct the model, the scientist has specified that the width of each cell at its widest point must be μ30μm and the height of each cell must be μ90 μm. Table 1 shows the three-dimensional shapes that the scientist is considering for the model cells. Which of the proposed shapes for the model cells will allow the most efficient exchange of materials with the surrounding environment?
Responses
A
Right circular cylinder
B
Triangular prism
C
Rectangular prism
D
Square-based pyramid
B
Triangular prism
A researcher measured the temperature at which two different samples of double-stranded DNA denature (separate into single strands). Sample 1 denatured at a significantly lower temperature than sample 2 did. Based on the data, the researcher claims that the DNA in sample 2 is composed of a higher percentage of guanine and cytosine than the DNA in sample 1 is.
Which of the following best supports the researcher’s claim?
Responses
A
The bonds between guanine and cytosine are covalent bonds, which require more energy to disrupt than those between adenine and thymine.
B
Guanine-cytosine pairs denature at a higher temperature because they have more hydrogen bonds between them than adenine-thymine pairs do.
C
Adenine-thymine pairs require less energy to separate because adenine and thymine are both single-ring bases.
D
Guanine-cytosine pairs require more energy to separate because one is a purine and one is a pyrimidine.
B
Guanine-cytosine pairs denature at a higher temperature because they have more hydrogen bonds between them than adenine-thymine pairs do.
Which of the following questions can best be answered by the diagram?
Responses
A
Does the addition of an enzyme reduce the activation energy required for a reaction?
B
Does the addition of an enzyme result in the formation of covalent bonds?
C
Does the addition of an enzyme produce a greater amount of products?
D
Does the addition of an enzyme change the pathway for the reaction?
A
Does the addition of an enzyme reduce the activation energy required for a reaction?
During respiration, most ATP is formed as a direct result of the net movement of
Responses
A
potassium against a concentration gradient
B
protons down a concentration gradient
C
electrons against a concentration gradient
D
electrons through a channel
E
sodium ions into the cell
B
protons down a concentration gradient
The energy required to run the Calvin cycle reactions of photosynthesis comes from which two substances produced during the light-dependent reactions?
Responses
A
ATP and NADPH
B
ADP and PO4
C
H+ and PO2
D
O2 and CO2
E
H2O and CO2
A
ATP and NADPH
The carbon 'that makes up organic molecules in plants is derived directly fromResponses
A
combustion of fuels
B
carbon fixed in photosynthesis
C
carbon dioxide produced in respiration
D
carbon in the lithosphere
E
coal mines
B
carbon fixed in photosynthesis
Which of the following best describes the function
of the
coenzymes NAD+ and FAD in eukaryotic
cellular respiration?
Responses
A
They participate in hydrolysis reactions by
accepting
protons from water molecules.
B
They participate directly in the
phosphorylation of ADP to ATP.
C
They serve as final electron acceptors in the
electron
transport chain.
D
They aid vitamins such as niacin in the breakdown
of glucose.
E
They accept electrons during oxidation-reduction
reactions.
E
They accept electrons during oxidation-reduction
reactions.
Which of the following best predicts which diagrammed microscope view the laboratory worker would see and best explains why?
Responses
A
View 1 because RBC membranes are freely permeable to water
B
View 2 because the RBCs use energy to allow sodium entry and to pump water out
C
View 2 because the rate of water movement into the RBCs equals the rate of water movement out of the cells
D
View 3 because the sodium-potassium pumps in the RBC membranes use energy to keep the sodium out but allow water to freely flow into the cells
C
View 2 because the rate of water movement into the RBCs equals the rate of water movement out of the cells
Which of the following is the best explanation for the pattern of change in mass of the organisms over time?
Responses
A
Water loss due to evaporation
B
Cellular respiration
C
The law of conservation of matter
D
Growth and reproduction
B
Cellular respiration
What is the most likely explanation for the change in the slope of the line between 3 and 5 minutes?
Responses
A
The enzyme had denatured.
B
The enzyme had achieved its maximum velocity.
C
A large amount of the substrate had been consumed.
D
An allosteric inhibitor appeared.
E
There was a dramatic change in the pH.
C
A large amount of the substrate had been consumed.
Which of the following best describes why the disks rose to the surface faster in the more concentrated hydrogen peroxide solutions?
Responses
A
There was more enzyme present in the more concentrated solutions.
B
A greater amount of heat was generated in the more concentrated solutions.
C
The more concentrated solutions lowered the activation energy of the reaction.
D
The higher substrate concentrations in the more concentrated solutions speeded the reaction.
E
The density of the water was lower in the more concentrated solutions.
D
The higher substrate concentrations in the more concentrated solutions speeded the reaction.
Which of the following best describes why ice was used during this experiment?
Responses
A
To increase the activity of the enzyme
B
To retard the breakdown of the catalase
C
To purge the solution of excess air trapped during blending
D
To slow the catalase molecules to increase the probability of contact with the hydrogen peroxide molecules
E
To increase the size of the active site on the enzyme
B
To retard the breakdown of the catalase
If the potato solution was boiled for 10 minutes and cooled for 10 minutes before being tested, the average time for the disks to float to the surface of the hydrogen peroxide solution would beResponses
A
less than 1 second
B
5 seconds
C
10 seconds
D
30 seconds
E
more than 30 seconds
E
more than 30 seconds
Bacteriophages are viruses that infect bacteria. In an experiment, bacteriophages were labeled with either radioactive phosphorus or radioactive sulfur. The labeled bacteriophages were incubated with bacteria for a brief amount of time and then removed. The infected bacteria cells were found to contain significant amounts of radioactive phosphorus but not radioactive sulfur.
Based on the results of the experiment, which of the following types of molecules did the bacteriophages most likely inject into the bacteria cells?
ResponsesA
Simple carbohydrate
B
Amino acid
C
DNA
D
Polypeptide
C
DNA
Which of the following can be used to determine the rate of enzyme-catalyzed reactions?
Responses
A
Rate of disappearance of the enzyme
B
Rate of disappearance of the substrate
C
Rate of disappearance of the product
D
Change in volume of the solution
E
Increase in activation energy
B
Rate of disappearance of the substrate
The rate of oxygen consumption in germinating pea seeds at 26ºC is
Responses
A
0.05 mL / min
B
0.25 mL / min
C
0.50 mL / min
D
0.75 mL / min
E
1.00 mL / min
A
0.05 mL / min
Which of the following conclusions is best supported by the data?
Responses
A
Nongerminating pea seeds have a higher rate of respiration than germinating pea seeds do.
B
Light is required for pea seed germination.
C
In the nongerminating pea seeds, oxygen consumption is directly proportional to oxygen concentration.
D
Less carbon dioxide is produced by germinating pea seeds at 26ºC than at 10ºC.
E
In pea seeds an increase in temperature results in an increase in oxygen consumption.
E
In pea seeds an increase in temperature results in an increase in oxygen consumption.
Which of the following statements is true regarding the movement of substances across cell membranes?
Responses
A
Ions are unable to move through the phospholipid bilayer because the nonpolar tail regions of the phospholipids are hydrophobic.
B
Ions are able to move through the phospholipid bilayer because the polar head regions of the phospholipids are charged.
C
Water is able to move through the phospholipid bilayer because the nonpolar tail regions of the phospholipids are charged.
D
Water is unable to move through the phospholipid bilayer because the polar head regions of the phospholipids are charged.
A
Ions are unable to move through the phospholipid bilayer because the nonpolar tail regions of the phospholipids are hydrophobic.
According to the results of this experiment, germination of tobacco seeds during the first week is
Responses
A
increased by exposure to light
B
unaffected by light intensity
C
prevented by paper towels
D
accelerated in green-leaved seedlings
A
increased by exposure to light
Additional observations were made on day 21, and no yellow-leaved seedlings were found alive in either dish. This is most likely because
Responses
A
yellow-leaved seedlings were unable to absorb water from the paper towels
B
taller green-leaved seedlings blocked the light and prevented photosynthesis
C
yellow-leaved seedlings were unable to convert light energy to chemical energy
D
a higher rate of respiration in yellow-leaved seedlings depleted their stored nutrients
C
yellow-leaved seedlings were unable to convert light energy to chemical energy
Based on the data shown, changes in the light intensity resulted in changes in the rate of which of the following processes?
Responses
A
Excretion
B
Photosynthesis
C
Respiration
D
Translation
E
Transcription
B
Photosynthesis
The rate of oxygen production equaled the rate of oxygen consumption during which of the following time periods?
Responses
A
G to H
B
H to I
C
I to J
D
J to K
E
G to K
D
J to K
An increase in the rate of oxygen production by algae would be accompanied by a comparable increase in the rate of production of which of the following substances?
Responses
A
C6H12O6
B
CO2
C
CH4
D
NH3
E
H2O
A
C6H12O6
Carbohydrate-synthesizing reactions of photosynthesis directly require
Responses
A
light
B
products of the light reactions
C
darkness
D
O2 and H2O
E
chlorophyll and CO2
B
products of the light reactions
Two nutrient solutions are maintained at the same pH. Actively respiring mitochondria are isolated and placed into each of the two solutions. Oxygen gas is bubbled into one solution. The other solution is depleted of available oxygen. Which of the following best explains why ATP production is greater in the tube with oxygen than in the tube without oxygen?
Responses
A
The rate of proton pumping across the inner mitochondrial membrane is lower in the sample without oxygen.
B
Electron transport is reduced in the absence of a plasma membrane.
C
In the absence of oxygen, oxidative phosphorylation produces more ATP than does fermentation.
D
In the presence of oxygen, glycolysis produces more ATP than in the absence of oxygen.
A
The rate of proton pumping across the inner mitochondrial membrane is lower in the sample without oxygen.
The fact that each line on the graph rises from left to right means that
Responses
A
higher temperatures produce higher rates of metabolism
B
there were more large fish in the samples taken at high temperatures
C
larger fish consume more oxygen than smaller fish at all four temperatures
D
when measurements are taken for larger fish late in the day, observed values are higher
E
larger fish prefer to live at higher temperatures than do smaller fish
C
larger fish consume more oxygen than smaller fish at all four temperatures
Which metabolic process is common to both aerobic cellular respiration and alcoholic fermentation?
Responses
A
Krebs cycle
B
Glycolysis
C
Electron transport chain
D
Conversion of pyruvic acid to acetyl CoA
E
Production of a proton gradient
B
Glycolysis
Paramecia are unicellular protists that have contractile vacuoles to remove excess intracellular water. In an experimental investigation, paramecia were placed in salt solutions of increasing osmolarity. The rate at which the contractile vacuole contracted to pump out excess water was determined and plotted against osmolarity of the solutions, as shown in the graph. Which of the following is the correct explanation for the data?
Responses
A
At higher osmolarity, lower rates of contraction are required because more salt diffuses into the paramecia.
B
The contraction rate increases as the osmolarity decreases because the amount of water entering the paramecia by osmosis increases.
C
The contractile vacuole is less efficient in solutions of high osmolarity because of the reduced amount of ATP produced from cellular respiration.
D
In an isosmotic salt solution, there is no diffusion of water into or out of the paramecia, so the contraction rate is zero.
B
The contraction rate increases as the osmolarity decreases because the amount of water entering the paramecia by osmosis increases.
Which of the following questions is most relevant to understanding the Calvin cycle?
Responses
A
How does chlorophyll capture light?
B
How is ATP used in the formation of 3-carbon carbohydrates?
C
How is NADP+ reduced to NADPH?
D
How is ATP produced in chemiosmosis?
B
How is ATP used in the formation of 3-carbon carbohydrates?
The rate of the reaction could also be determined by
Responses
A
measuring the change in the amount of enzyme
B
measuring the change in the amount of substrate
C
measuring the change in salt concentration
D
adding more substrate
E
adding more enzyme
B
measuring the change in the amount of substrate
According to the chemiosmotic theory (chemiosmotic coupling), the energy required to move protons from the mitochondrial matrix to the intermembrane space against a concentration gradient comes most directly from
Responses
A
photons of red or blue light
B
the hydrolysis of ATP
C
the breakdown of high-energy fatty acids in
the
mitochondrial matrix
D
electrons flowing along the electron transport
chain
E
substrate-level phosphorylation
D
electrons flowing along the electron transport
chain
A human kidney filters about 200 liters of blood each day. Approximately two liters of liquid and nutrient waste are excreted as urine. The remaining fluid and dissolved substances are reabsorbed and continue to circulate throughout the body. Antidiuretic hormone (ADH) is secreted in response to reduced plasma volume. ADH targets the collecting ducts in the kidney, stimulating the insertion of aquaporins into their plasma membranes and an increased reabsorption of water.
If ADH secretion is inhibited, which of the following would initially result?
Responses
A
The number of aquaporins would increase in response to the inhibition of ADH.
B
The person would decrease oral water intake to compensate for the inhibition of ADH.
C
Blood filtration would increase to compensate for the lack of aquaporins.
D
The person would produce greater amounts of dilute urine
D
The person would produce greater amounts of dilute urine
ATP serves as a common energy source for organisms because
Responses
A
it is the smallest energy molecule
B
it stores the least energy of any energy source
C
its energy can be easily transferred to do cellular work
D
it is extremely stable and can be stored in the cell for long periods of time
E
traces of it have been found in fossils of ancient organisms dating back to the beginning of life on Earth
C
its energy can be easily transferred to do cellular work
Which of the following statements about mitochondrial chemiosmosis is NOT true?
Responses
A
A proton gradient is established across the inner membrane of the mitochondrion.
B
The potential energy released from the mitochondrial proton gradient is used to produce ATP.
C
The mitochondrial proton gradient provides energy for muscle contraction.
D
Proteins embedded in the inner mitochondrial membrane play an important role in ATP synthesis.
E
Heat energy is required to establish the electron transport chain.
E
Heat energy is required to establish the electron transport chain.
Figure 1. Diagram of the electron transport chain and ATP synthase in the membrane of mitochondria
On average, more ATP can be produced from an NADH molecule than can be produced from a molecule of FADH2. Based on Figure 1, which of the following best explains the difference in ATP production between these two molecules?
Responses
A
NADH contributes more electrons to the electron transport chain than FADH2 does and therefore provides more energy to pump protons.
B
The electrons of FADH2 are transferred through three complexes of the electron transport chain whereas those of NADH are transferred through all four complexes.
C
FADH2 contributes more protons to the mitochondrial matrix, which decreases the proton gradient.
D
The protons contributed by FADH2 are combined with �2 to make water and are not pumped across the membrane.
B
The electrons of FADH2 are transferred through three complexes of the electron transport chain whereas those of NADH are transferred through all four complexes.
Which of the following is an important difference between light-dependent and light-independent reactions of photosynthesis?
Responses
A
The light-dependent reactions occur only during the day; the light-independent reactions occur only during the night.
B
The light-dependent reactions occur in the cytoplasm; the light-independent reactions occur in chloroplasts.
C
The light-dependent reactions utilize CO2 and H2O; the light-independent reactions produce CO2 and H2O.
D
The light-dependent reactions depend on the presence of both photosystems I and II; the light-independent reactions require only photosystem I.
E
The light-dependent reactions produce ATP and NADPH; the light-independent reactions use energy stored in ATP and NADPH.
E
The light-dependent reactions produce ATP and NADPH; the light-independent reactions use energy stored in ATP and NADPH.
It is estimated that oxygen production first evolved in photosynthetic prokaryotes approximately 2.7 billion years ago. The first photosynthetic prokaryotes are presumed to be similar to today’s cyanobacteria.
Which of the following best supports the claim that photosynthetic prokaryotes were responsible for the oxygen in Earth’s atmosphere?
Responses
A
The light reactions of photosynthesis split carbon dioxide into carbon and oxygen.
B
The light reactions of photosynthesis split water into hydrogen ions and oxygen.
C
The Calvin cycle splits glucose into carbon, hydrogen, and oxygen.
D
The Calvin cycle splits water into hydrogen ions and oxygen.
B
The light reactions of photosynthesis split water into hydrogen ions and oxygen.
A tissue culture of vertebrate muscle was provided with a constant excess supply of glucose under anaerobic conditions starting at time zero and the amounts of pyruvic acid and ATP produced were measured. The solid line in the graph above represents the pyruvic acid produced in moles per liter per minute. ATP levels were also found to be highest at points A and C, lowest at B and D. A second culture was set up under the same conditions, except that substance X was added, and the results are indicated by the dotted line.
Which of the following best accounts for the shape of the solid line between points A and D?
Responses
A
After ten minutes the cellular enzymes became ineffective.
B
Respiration became uncontrolled.
C
ATP acted as an allosteric inhibitor on one or more of the enzymes.
D
The measurements of pyruvic acid were unreliable.
E
The cells required more glucose than was being provided.
C
ATP acted as an allosteric inhibitor on one or more of the enzymes.
A tissue culture of vertebrate muscle was provided with a constant excess supply of glucose under anaerobic conditions starting at time zero and the amounts of pyruvic acid and ATP produced were measured. The solid line in the graph above represents the pyruvic acid produced in moles per liter per minute. ATP levels were also found to be highest at points A and C, lowest at B and D. A second culture was set up under the same conditions, except that substance X was added, and the results are indicated by the dotted line.
It is most reasonable to hypothesize that, in the breakdown of glucose, substance X is
Responses
A
an activator
B
an inhibitor
C
a substrate
D
a coenzyme
E
a cofactor
B
an inhibitor
A tissue culture of vertebrate muscle was provided with a constant excess supply of glucose under anaerobic conditions starting at time zero and the amounts of pyruvic acid and ATP produced were measured. The solid line in the graph above represents the pyruvic acid produced in moles per liter per minute. ATP levels were also found to be highest at points A and C, lowest at B and D. A second culture was set up under the same conditions, except that substance X was added, and the results are indicated by the dotted line.
Which of the following is most likely to result if oxygen is added to the tissue culture?
Responses
A
Lactic acid formation will increase.
B
For each glucose molecule consumed, more ATP will be formed.
C
The levels of ATP produced will decrease.
D
Ethyl alcohol will be produced.
E
No change in the production of pyruvic acid will be observed.
B
For each glucose molecule consumed, more ATP will be formed.
Ethylene is an organic compound produced by ripening fruits. In a controlled experiment, researchers found that ethylene gas stimulated the ripening process in newly harvested fruits. Which of the following describes the most likely connection between natural ethylene production and fruit ripening?
Responses
A
As a result of metabolic inactivity, newly harvested fruits are unable to absorb ethylene gas from the atmosphere.
B
Ethylene gas is a chemical signal through which ripening fruits trigger the ripening process in other fruits.
C
Because of normal phenotypic variation, only some of the fruits in a given generation are expected to produce ethylene gas.
D
The rate of ethylene gas production by ripening fruits is an indicator of the relative age of an ecosystem.
Related Content & Skills
- Topic4.1
- Skill6.A
Related Videos4.1: Daily Video 1
B
Ethylene gas is a chemical signal through which ripening fruits trigger the ripening process in other fruits.
Epinephrine is a protein hormone found in many animals. Epinephrine stimulates a signaling pathway that results in the breakdown of glycogen to glucose in the liver cells. Which of the following describes the initial steps in the process whereby epinephrine stimulates glycogen breakdown?
Responses
A-
Epinephrine binds to a cell-surface receptor; the activated receptor stimulates production of the second messenger, cAMP.
B
Epinephrine binds to a cell-surface receptor; the activated receptor catalyzes the conversion of glycogen to glucose.
C
Epinephrine diffuses through the plasma membrane; the hormone dimerizes in the cytosol.
D
Epinephrine is taken into the cell by endocytosis; glycogen is converted to glucose in the endocytotic vesicle.
A-
Epinephrine binds to a cell-surface receptor; the activated receptor stimulates production of the second messenger, cAMP.
It is estimated that oxygen production first evolved in photosynthetic prokaryotes approximately 2.7 billion years ago. The first photosynthetic prokaryotes are presumed to be similar to today’s cyanobacteria.
Which of the following best supports the claim that photosynthetic prokaryotes were responsible for the oxygen in Earth’s atmosphere?
Responses
A
The light reactions of photosynthesis split carbon dioxide into carbon and oxygen.
B
The light reactions of photosynthesis split water into hydrogen ions and oxygen.
C
The Calvin cycle splits glucose into carbon, hydrogen, and oxygen.
D
The Calvin cycle splits water into hydrogen ions and oxygen.
B
The light reactions of photosynthesis split water into hydrogen ions and oxygen.
Figure 2: Blood insulin levels in normal mice and ��� mutant mice after exposure to glucose
Hormones are chemical signals that are released by cells in one part of the body that travel through the bloodstream to signal cells in another part of the body. Insulin is a hormone that is released by the pancreas that induces the uptake of glucose molecules from the bloodstream into cells. In this way, insulin lowers the overall blood glucose levels of the body. Osteoblasts and osteoclasts are two types of bone cells that play a role in regulating blood glucose levels (Figure 1).
Binding of insulin to the insulin receptor on osteoblasts activates a signaling pathway that results in osteoblasts releasing a molecule, OPG, that binds to neighboring osteoclasts. In response, the osteoclasts release protons (H+) and create an area of lower pH outside the cell. This low pH activates osteocalcin, a protein secreted in an inactive form by osteoblasts.
The ��� gene encodes a protein that alters the structure of the insulin receptor on osteoblasts and interferes with the binding of insulin to the receptor. A researcher created a group of osteoblasts with an ��� mutation that prevented the production of a functional ��� product (mutant). The researcher then exposed the mutant strain and a normal strain that expresses ��� to glucose and compared the levels of insulin in the blood near the osteoblasts (Figure 2).
Which of the following best describes the effect of insulin binding to the receptor on the osteoblast cells?
Responses
A
Insulin binding ultimately increases pancreatic secretion of additional insulin.
B
Insulin binding blocks the release of osteocalcin from the osteoblasts.
C
Insulin binding inhibits the expression of ���.
D
Insulin binding increases the pH of the extracellular matrix.
A
Insulin binding ultimately increases pancreatic secretion of additional insulin.
Based on the information provided, which of the following best justifies the claim that osteocalcin is a hormone?
Responses
A
The phosphorylation of the insulin receptor causes a response in osteoblast bone cells.
B
The osteoblasts in the bone secrete osteocalcin, which causes cells in the pancreas to change their activity.
C
The change in expression of ��� changes the insulin receptor activity of the osteoblast.
D
The activation of the osteocalcin by a bone cell is pH dependent.
B
The osteoblasts in the bone secrete osteocalcin, which causes cells in the pancreas to change their activity.
Which of the following claims is most consistent with the data shown in Figure 2 ?
Responses
A
Esp expression is necessary to prevent the overproduction of insulin.
B
Esp protein does not regulate blood-sugar levels in normal mice.
C
Normal mice require a higher blood concentration of insulin than mutant mice do.
D
Mutant mice have a cyclical pattern of insulin secretion.
A
Esp expression is necessary to prevent the overproduction of insulin.
Which of the following was a positive control in the experiment?
Responses
A
Minutes after glucose injection
B
Blood insulin
C
Mutant strain
D
Normal strain
D
Normal strain
A researcher observes that mice from the mutant strain experience low blood sugar. Which of the following best describes the feedback mechanism in the pathway (Figure 1) causing the low blood sugar in the mutant strain?
Responses
A
The positive feedback of insulin production
B
The negative feedback of inactive osteocalcin production
C
The positive feedback of the Esp protein
D
The negative feedback of insulin-secreting pancreatic cell proliferation
A
The positive feedback of insulin production
The mechanism of action of many common medications involves interfering with the normal pathways that cells use to respond to hormone signals. Which of the following best describes a drug interaction that directly interferes with a signal transduction pathway?
Responses
A
A medication causes the cell to absorb more of a particular mineral, eventually poisoning the cell.
B
A medication enters the target cell and inhibits an enzyme that normally synthesizes a second messenger.
C
A medication enters the target cell’s nucleus and acts as a mutagen.
D
A medication interrupts the transcription of ribosomal RNA genes.
B
A medication enters the target cell and inhibits an enzyme that normally synthesizes a second messenger.
Figure 1. Diagram of the electron transport chain and ATP synthase in the membrane of mitochondria
On average, more ATP can be produced from an NADH molecule than can be produced from a molecule of FADH2. Based on Figure 1, which of the following best explains the difference in ATP production between these two molecules?
Responses
A
NADH contributes more electrons to the electron transport chain than FADH2 does and therefore provides more energy to pump protons.
B
The electrons of FADH2 are transferred through three complexes of the electron transport chain whereas those of NADH are transferred through all four complexes.
C
FADH2 contributes more protons to the mitochondrial matrix, which decreases the proton gradient.
D
The protons contributed by FADH2 are combined with �2 to make water and are not pumped across the membrane.
B
The electrons of FADH2 are transferred through three complexes of the electron transport chain whereas those of NADH are transferred through all four complexes.
Which of the following is a valid interpretation of the experimental results that explains how individuals with type 2 diabetes differ from individuals without diabetes?
Responses
A
The relatively low levels of glucose uptake in individuals with type 2 diabetes indicate that mobilization of GLUT4 to the cell surface is reduced in muscle cells of those individuals.
B
The relatively low levels of glucose uptake in individuals with type 2 diabetes indicate that no functional GLUT4 protein is produced in the muscle cells of those individuals.
C
The absence of activated insulin receptors in individuals with type 2 diabetes indicates that no insulin is secreted by the pancreatic cells of those individuals.
DThe absence of activated IRS-1 in individuals with type 2 diabetes indicates that no functional insulin receptor protein is produced in the muscle cells of those individuals.
A
The relatively low levels of glucose uptake in individuals with type 2 diabetes indicate that mobilization of GLUT4 to the cell surface is reduced in muscle cells of those individuals.
Based on the experimental results, which of the following describes the most likely defect in muscle cells of patients with type 2 diabetes?
Responses
A
Insulin receptor proteins do not reach the cell surface.
B
Insulin does not activate its receptor.
C
IRS-1 activation is reduced at high insulin concentrations.
D
GLUT4 blocks glucose from entering cells.
C
IRS-1 activation is reduced at high insulin concentrations.
Based on the information presented, which of the following genetic changes in an individual without diabetes is most likely to result in a disrupted cellular response to insulin signaling similar to that of an individual with type 2 diabetes?
Responses
A
A deletion in the gene encoding the insulin receptor that removes only the cytoplasmic domain of the protein
B
Duplication of the gene encoding a PI-3 kinase that results in synthesis of a muscle-specific variant of the enzyme in skin cells as well as in muscle cells
C
A mutation in the gene encoding IRS-1 that causes the protein to be active in muscle cells even in the absence of insulin signaling
DInsertion of a small segment of DNA into the promoter of the Glut4 gene that results in increased synthesis of GLUT4 proteins in muscle cells
A
A deletion in the gene encoding the insulin receptor that removes only the cytoplasmic domain of the protein
Beta cells, a type of cell found in the pancreas, are responsible for producing, storing, and releasing the hormone (chemical signal) insulin. An increase in blood glucose levels stimulates beta cells to produce insulin, which facilitates the uptake of glucose by body cells and reduces the glucose levels in the blood. As the blood glucose levels decrease, beta cells are no longer stimulated and stop producing insulin.
Which of the following best explains the feedback mechanism that regulates blood glucose levels?
Responses
A
Regulation of blood glucose levels demonstrates positive feedback, because as the blood glucose levels increase, insulin acts to raise blood glucose levels.
B
Regulation of blood glucose levels demonstrates negative feedback, because as the blood glucose levels increase, insulin acts to lower blood glucose levels.
C
Regulation of blood glucose levels demonstrates positive feedback, because as the production of insulin by beta cells increases, blood glucose levels increase.
D
Regulation of blood glucose levels demonstrates negative feedback, because as the production of insulin by beta cells decreases, blood glucose levels decrease.
B
Regulation of blood glucose levels demonstrates negative feedback, because as the blood glucose levels increase, insulin acts to lower blood glucose levels.
Which of the following best describes the function
of the
coenzymes NAD+ and FAD in eukaryotic
cellular respiration?
Responses
A
They participate in hydrolysis reactions by
accepting
protons from water molecules.
B
They participate directly in the
phosphorylation of ADP to ATP.
C
They serve as final electron acceptors in the
electron
transport chain.
D
They aid vitamins such as niacin in the breakdown
of glucose.
E
They accept electrons during oxidation-reduction
reactions.
E
They accept electrons during oxidation-reduction
reactions.
If chemical signals in the cytoplasm control the progression of a cell to the M phase of the cell cycle, then fusion of a cell in G1 with a cell in early M phase would most likely result in the
Responses
A
replication of chromosomes only in the G1 cell
B
exiting of both cells from the cell cycle and into the G0 phase
C
condensation of chromatin in preparation of nuclear division in both cells
D
transfer of organelles from the G1 cell to the cell in the M phase
C
condensation of chromatin in preparation of nuclear division in both cells
The drug 5-fluorouracil inhibits thymine production in eukaryotic cells. Which of the following cell cycle stages will be most directly affected by 5-fluorouracil?
Responses
A
The first growth phase (G1)
B
Synthesis of DNA phase (S)
C
Preparation for mitosis (G2)
D
Cytokinesis
B
Synthesis of DNA phase (S)
Two nutrient solutions are maintained at the same pH. Actively respiring mitochondria are isolated and placed into each of the two solutions. Oxygen gas is bubbled into one solution. The other solution is depleted of available oxygen. Which of the following best explains why ATP production is greater in the tube with oxygen than in the tube without oxygen?
Responses
A
The rate of proton pumping across the inner mitochondrial membrane is lower in the sample without oxygen.
B
Electron transport is reduced in the absence of a plasma membrane.
C
In the absence of oxygen, oxidative phosphorylation produces more ATP than does fermentation.
D
In the presence of oxygen, glycolysis produces more ATP than in the absence of oxygen.
A
The rate of proton pumping across the inner mitochondrial membrane is lower in the sample without oxygen.
A researcher examining a root tip observes a plant cell with condensed sister chromatids, kinetochores with attached microtubules, and individual chromosomes that are aligned at the equatorial plate of the cell. Which of the following best describes what the next process will be in the cell?
Responses
A
Homologous chromosomes (each with two sister chromatids) will move toward opposite poles of the cell.
B
Paired chromatids will separate, and the new daughter chromosomes will move toward opposite poles of the cell.
C
The nuclear envelope will break down, and the spindle will begin to form.
D
The chromatin will decondense, and the daughter cell will enter interphase.
B
Paired chromatids will separate, and the new daughter chromosomes will move toward opposite poles of the cell.
Which of the following is true of mitosis?
Responses
A
It is also known as cytokinesis.
B
It maintains the same chromosome number in the daughter cells as in the parent cell.
C
It is the last phase of interphase.
D
It regulates the transfer of genetic information from one daughter cell to another.
B
It maintains the same chromosome number in the daughter cells as in the parent cell.
Which of the following best explains the most likely method by which this antitumor drug works?
Responses
A
Trabectedin increases the production of cyclin proteins that signal the cancer cells to enter prophase.
B
Trabectedin interferes with the plasma membrane, causing it to break down and expose the DNA to damage.
C
Trabectedin interferes with the duplication of DNA during interphase and thus prevents cancer cells from passing the G2 checkpoint.
D
Trabectedin interferes with the regulations of cyclin proteins, causing their levels to increase and creating errors in DNA.
C
Trabectedin interferes with the duplication of DNA during interphase and thus prevents cancer cells from passing the G2 checkpoint.
Figure 1. The relative concentrations of both the cyclin and CDK components of MPF
Maturation promoting factor, MPF, is a cyclin-CDK complex that catalyzes the phosphorylation of other proteins to start mitosis. The activity level of MPF is dependent on the relative concentrations of the cyclin and CDK components of MPF (Figure 1).
Based on Figure 1, which of the following describes the role of cyclin in the regulation of the cell cycle?
Responses
A
During G1 phase, the cyclin level decreases to signal the start of the resting phase of the cell cycle.
B
During M phase, the cyclin level peaks, resulting in an increased binding frequency with CDK.
C
During S phase, the cyclin level remains the same because DNA replication is occurring.
D
During G2 phase, the cyclin level remains low, causing MPF activity to decrease, which leads cells to initiate mitosis.
B
During M phase, the cyclin level peaks, resulting in an increased binding frequency with CDK.
Which of the following is an important difference between light-dependent and light-independent reactions of photosynthesis?
Responses
A
The light-dependent reactions occur only during the day; the light-independent reactions occur only during the night.
B
The light-dependent reactions occur in the cytoplasm; the light-independent reactions occur in chloroplasts.
C
The light-dependent reactions utilize CO2 and H2O; the light-independent reactions produce CO2 and H2O.
D
The light-dependent reactions depend on the presence of both photosystems I and II; the light-independent reactions require only photosystem I.
E
The light-dependent reactions produce ATP and NADPH; the light-independent reactions use energy stored in ATP and NADPH.
E
The light-dependent reactions produce ATP and NADPH; the light-independent reactions use energy stored in ATP and NADPH.
One approach to treating patients with pancreatic cancer and other cancers in which the Hedgehog protein is detected is to modify the Hedgehog signaling pathway. Which of the following is the most useful approach?
Responses
A
Treating patients with a molecule that is structurally similar to Hedgehog and that will bind to and interact with Ptc in the same fashion as Hedgehog
B
Injecting patients with embryonic cells so that Hedgehog will bind to those cells instead of the cancer cells
C
Treating patients with a membrane-soluble compound that can bind to Smo and block its activity
D
Injecting patients with a preparation of purified membrane-soluble Ci that will enter the nuclei of the cancer cells and induce gene transcription
C
Treating patients with a membrane-soluble compound that can bind to Smo and block its activity
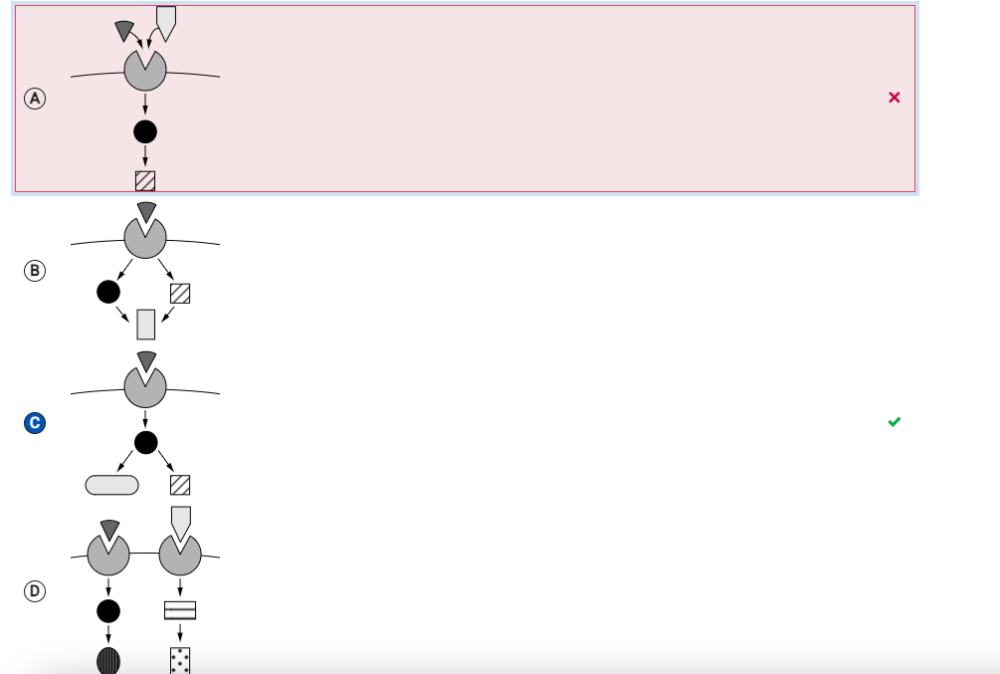
Which of the following best represents a signaling pathway that will lead to multiple responses from the same signaling molecule?
A
B
C
D
C
Figure 1. A model of an endocrine signaling pathway showing involved body parts and hormones. GnRH = gonadotropin-releasing hormone, LH = luteinizing hormone, and FSH = follicle-stimulating hormone.
Figure 1 shows a model of the endocrine signaling pathway that regulates ovulation. Which of the following observations would provide evidence of a positive feedback mechanism in this system?
Responses
A
Estrogen from the ovaries inhibits the release of GnRH from the hypothalamus.
B
Progesterone from the ovaries stimulates the thickening of the uterine lining.
C
Progesterone from the ovaries inhibits the release of LH and FSH from the anterior pituitary.
D
Estrogen from the ovaries stimulates the hypothalamus and anterior pituitary to secrete more GnRH, LH, and FSH.
D
Estrogen from the ovaries stimulates the hypothalamus and anterior pituitary to secrete more GnRH, LH, and FSH.