A mole is a counting number that means 6.022 × 10 23 just as a dozen means 12. We can create a conversion factor that can be used to determine the number of particles in a mole or vice versa.
6.022 x 1023 particles / 1 mole
OR
1 mole / 6.022 x 1023 particles
The chemical formula of a compound gives the ratio of the elements in that compound. The subscripts can be used as a ratio of atoms or moles. The ratio can be used to convert between atoms and molecules.
The molar mass has units of grams per mole and can be used as a conversion factor between the grams and moles of a substance. The numerical value for the relationship between grams and moles will vary depending on the identity of the substance.
grams / mole
OR
mole / grams
How many atoms of S in 4.30 grams of CS 2 ?
- 1.70 × 1022 atoms
- 3.40 × 1022 atoms
- 6.80 × 1022 atoms
- 3.94 × 1026 atoms
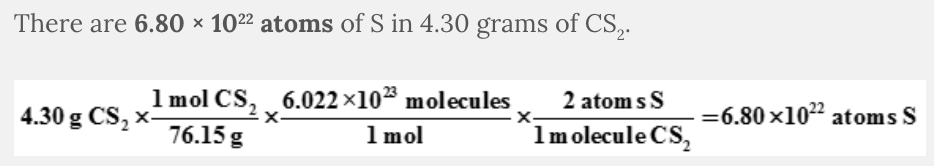
6.80 × 10 22 atoms
Molar mass is the mass of 1 mole, in grams, of a given substance. This results in the units of g/mol for molar mass. The number of grams is determined by adding up the atomic masses of the individual elements on the periodic table.
Molar mass is a conversion factor that can be used to determine the number of moles in a given number of grams or vice versa. The conversion factor for molar mass would be the following:
g / 1 mole
OR
1 mole / g
How many moles of butane, C 4 H 10 , are found in 392.3 grams of C 4 H 10 ?
- 4.386 mol C4H10
- 6.750 mol C4H10
- 2.280 mol C4H10
- 0.1482 mol C4H10
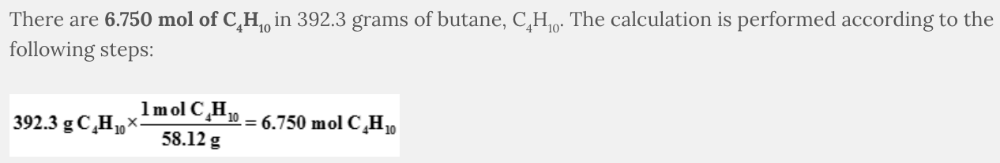
6.750 mol C 4 H 10
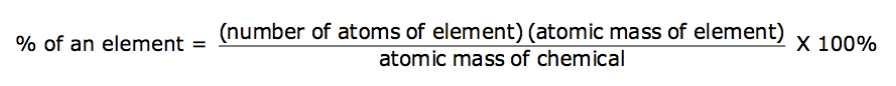
Mass percent composition involves calculating the percentage of mass for each of the individual elements in a given substance.
Mass percent composition is calculated according to the following equation:
image attached
The percentages for each of the individual elements will then add up to 100%.
Which element has a mass percent composition of 21.49% in borax, Na 2 B 4 O 7 ?
- O
- B
- Na
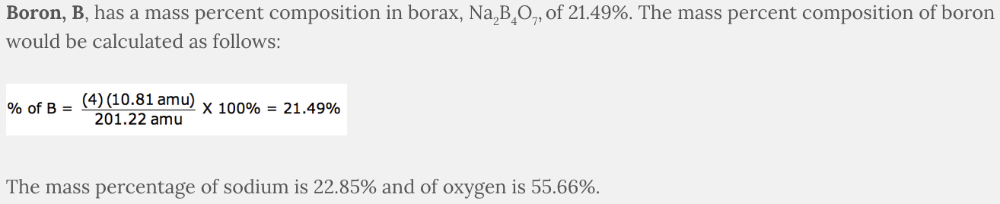
B
Molar mass is the mass of 1 mole, in grams, of a given substance. This results in the units of g/mol for molar mass. The number of grams is determined by adding up the atomic masses of the individual elements on the periodic table.
Which substance has a molar mass of 33.99 g/mol?
- GaF3
- PH3
- SF2
- CO2
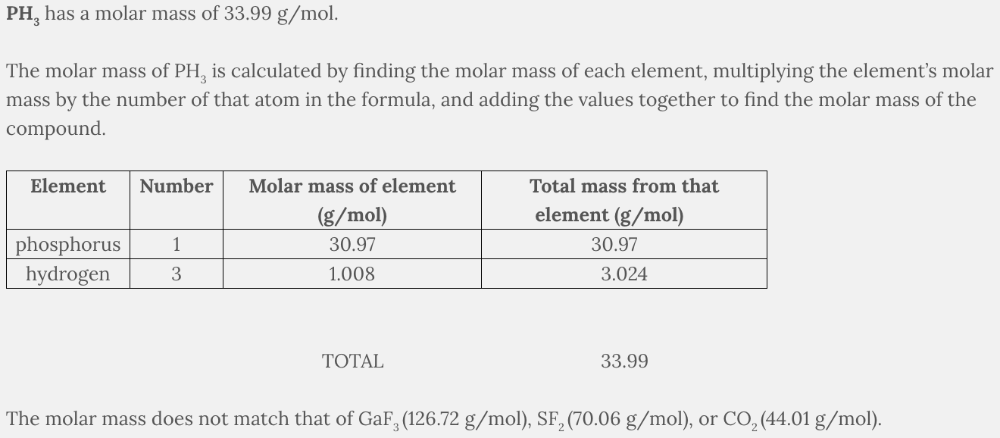
PH 3
A mole is a counting number that means 6.022 × 10 23 just as a dozen means 12. We can create a conversion factor that can be used to determine the number of particles in a mole or vice versa.
6.022 x 1023 particles / 1 mole
OR
1 mole / 6.022 x 1023 particles
The chemical formula of a compound gives the ratio of the elements in that compound. The subscripts can be used as a ratio of atoms or moles. The ratio can be used to convert between atoms and molecules.
How many atoms of H are in 2.8 mol N 2 H 4 ?
- 6.7 × 1024 atoms
- 1.7 × 1024 atoms
- 5.9 × 1025 atoms
- 4.2 × 1023 atoms
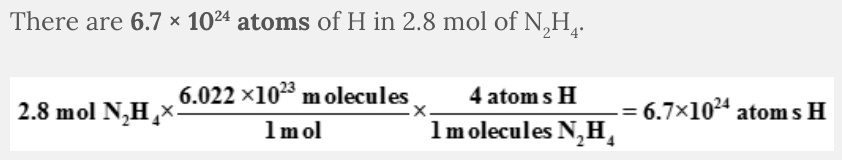
6.7 × 10 24 atoms
The empirical formula is a chemical formula that provides the smallest, whole-number ratio of elements in a compound. Using the mass of each element or the mass percentage of each element in a compound, the empirical formula can be determined.
What is the empirical formula for a substance containing 0.0923 grams of carbon, C, and 0.0077 grams of hydrogen, H?
- C3H6
- C2H2
- CH4
- CH

CH
The empirical formula is a chemical formula that provides the smallest, whole-number ratio of elements in a compound. Using the mass of each element or the mass percentage of each element in a compound, the empirical formula can be determined.
The molecular formula is a whole-number multiple of the empirical formula. The multiple is determined from the ratio of the molar mass to the empirical formula mass.
Whole-number multiple = molar mass / empirical formula mass
What is the molecular formula if the molar mass is 168.0 g/mol and the empirical formula is C 4 H 4 S?
- C4H4S
- C8H8S2
- C4H4S2
- C6H6S
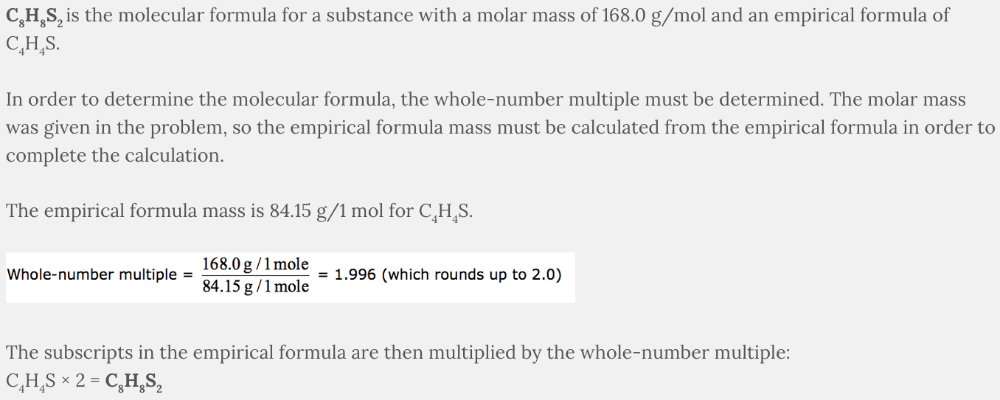
C 8 H 8 S 2
The chemical formula of a compound gives the ratio of the elements in that compound. The subscripts can be used as a ratio of atoms or moles. The ratio can be used to convert between atoms and molecules.
How many atoms of hydrogen are in 3.02 × 10 22 molecules of NH 3 ?
- 9.06 × 1022 atoms
- 2.72 × 1022 atoms
- 1.01 × 1022 atoms
- 3.02 × 1022 atoms
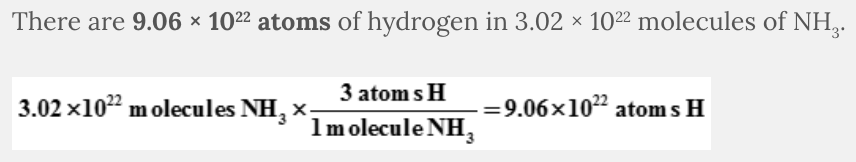
9.06 × 10 22 atoms
The empirical formula is a chemical formula that provides the smallest, whole-number ratio of elements in a compound. Using the mass of each element or the mass percentage of each element in a compound, the empirical formula can be determined.
What is the empirical formula for a substance containing 28.6 grams of magnesium, Mg, 14.3 grams of carbon, C, and 57.1 grams of oxygen, O?
- MgCO
- Mg2C2O6
- MgCO3
- MgC3O

MgCO 3
Mole is a Latin word for “pile.” More specifically, a mole is a pile that contains 6.022 x 10 23 particles or objects. These particles are typically atoms, molecules, or ions. A mole is a counting number that means 6.022 × 10 23 just as a dozen means 12.
With this in mind, we can now create a conversion factor that can be used to determine the number of particles in a mole (or “pile”) or vice versa.
6.022 x 1023 particles / 1 mole
OR
1 mole / 6.022 x 1023 particles
When using this conversion factor, you will replace “particles” with the chemical identity of the substance you are using, as well as identifying if the substance is found as atoms, molecules, or ions.
Calculate the number of moles of water, H 2 O, if you begin with 3.7 x 10 24 molecules of water.
- 0.16 moles of H2O
- 6.1 moles of H2O
- 6.1 x 1046 moles of H2O
- 1.6 × 10-47 moles of H2O
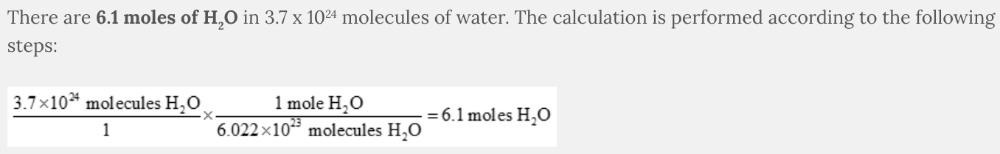
6.1 moles of H 2 O
Molar mass is the mass of 1 mole, in grams, of a given substance. This results in the units of g/mol for molar mass. The number of grams is determined by adding up the atomic masses of the individual elements on the periodic table.
What is the molar mass of acetone, C 3 H 6 O?
- 53.04 g/mol
- 58.08 g/mol
- 31.03 g/mol
- 29.02 g/mol
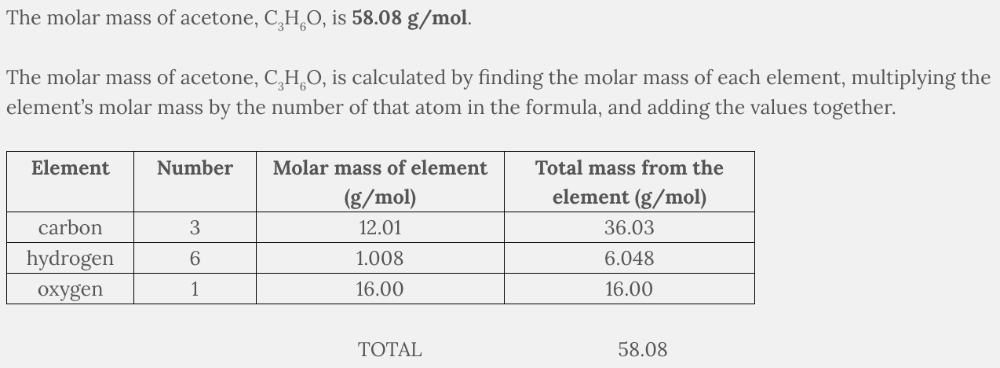
58.08 g/mol
The empirical formula is a chemical formula that provides the smallest, whole-number ratio of elements in a compound. Using the mass of each element or the mass percentage of each element in a compound, the empirical formula can be determined.
What is the empirical formula for a substance that contains 50.05% sulfur and 49.95% oxygen by mass?
- SO2
- SO3
- SO
- S2O

SO 2
Molar mass is the mass of 1 mole, in grams, of a given substance. This results in the units of g/mol for molar mass. The number of grams is determined by adding up the atomic masses of the individual elements on the periodic table.
Molar mass is a conversion factor that can be used to determine the number of moles in a given number of grams or vice versa. The conversion factor for molar mass would be the following:
g / 1 mole
OR
1 mole / g
How many grams of diethyl ether, C 4 H 10 O, are found in 5.81 mol of C 4 H 10 O?
- 0.0784 g of C4H10O
- 12.8 g of C4H10O
- 0.00232 × 10-3 g of C4H10O
- 431 g of C4H10O
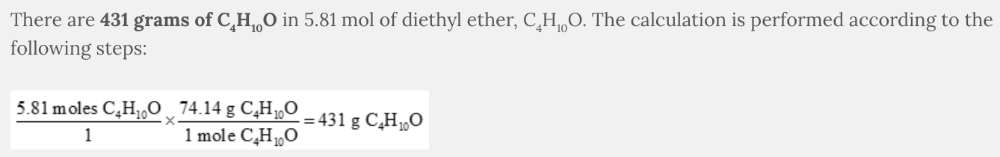
431 g of C 4 H 10 O
A mole is a counting number that means 6.022 × 10 23 just as a dozen means 12. We can create a conversion factor that can be used to determine the number of particles in a mole or vice versa.
6.022 x 1023 particles / 1 mole
OR
1 mole / 6.022 x 1023 particles
The chemical formula of a compound gives the ratio of the elements in that compound. The subscripts can be used as a ratio of atoms or moles. The ratio can be used to convert between atoms and molecules.
The molar mass has units of grams per mole and can be used as a conversion factor between the grams and moles of a substance. The numerical value for the relationship between grams and moles will vary depending on the identity of the substance.
grams / mole
OR
mole / grams
How many grams of nitrogen are in 13.2 grams of N 2 O 4 ?
- 1.00 g
- 4.02 g
- 2.01 g
- 4.33 g
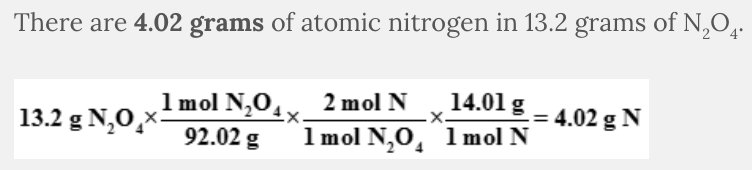
4.02 g
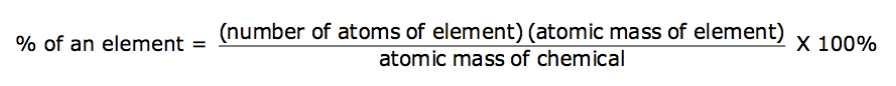
Mass percent composition involves calculating the percentage of mass for each of the individual elements in a given substance.
Mass percent composition is calculated according to the following equation:
image attached
The percentages for each of the individual elements will then add up to 100%.
What is the mass percent composition of calcium in calcium sulfate, CaSO 4 ?
- 100.0%
- 47.00%
- 23.55%
- 29.44%

29.44%
A mole is a counting number that means 6.022 × 10 23 just as a dozen means 12. We can create a conversion factor that can be used to determine the number of particles in a mole or vice versa.
6.022 x 1023 particles / 1 mole
OR
1 mole / 6.022 x 1023 particles
The molar mass has units of grams per mole and can be used as a conversion factor between the grams and moles of a substance. The numerical value for the relationship between grams and moles will vary depending on the identity of the substance.
grams / mole
OR
mole / grams
How many grams of H 2 S are found in a sample with 5.03 × 10 24 molecules of H 2 S?
- 408 g
- 285 g
- 245 g
- 351 g
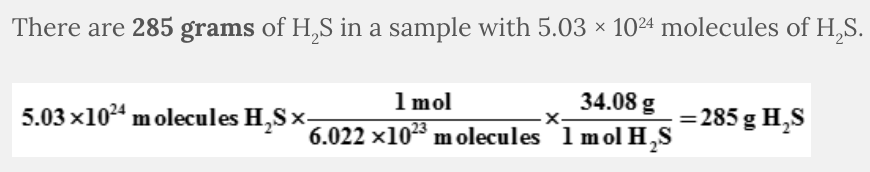
285 g
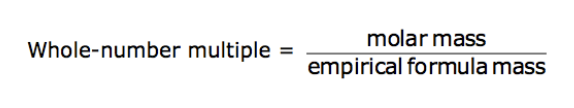
The empirical formula is a chemical formula that provides the smallest, whole-number ratio of elements in a compound. Using the mass of each element or the mass percentage of each element in a compound, the empirical formula can be determined.
The molecular formula is a whole-number multiple of the empirical formula. The multiple is determined from the ratio of the molar mass to the empirical formula mass.
image attached
What is the molecular formula if the molar mass is 34.02 g/mol and the empirical formula is HO?
- HO
- H2O2
- H2O
- HO2

H 2 O 2
The empirical formula is a chemical formula that provides the smallest, whole number ratio of elements in a compound. Using the mass of each element or the mass percentage of each element in a compound, the empirical can be determined.
What is the empirical formula for a substance that contains 1.003% hydrogen, 35.29% chlorine, and 63.71% oxygen by mass?
- HClO2
- HCl2O2
- HClO3
- HClO4

HClO 4