Calculate ∆H rxn for 2 NOCl( g ) → N 2 ( g ) + O 2 ( g ) + Cl 2 ( g ) given the following:
½ N 2 ( g ) + ½ O 2 ( g ) → NO( g ) ∆H rxn = 90.3 kJ
NO( g ) + ½ Cl 2 ( g ) → NOCl( g ) ∆H rxn = −38.6 kJ
- 103.4 kJ
- 51.7 kJ
- −51.7 kJ
- −103.4 kJ
−103.4 kJ
Ex.
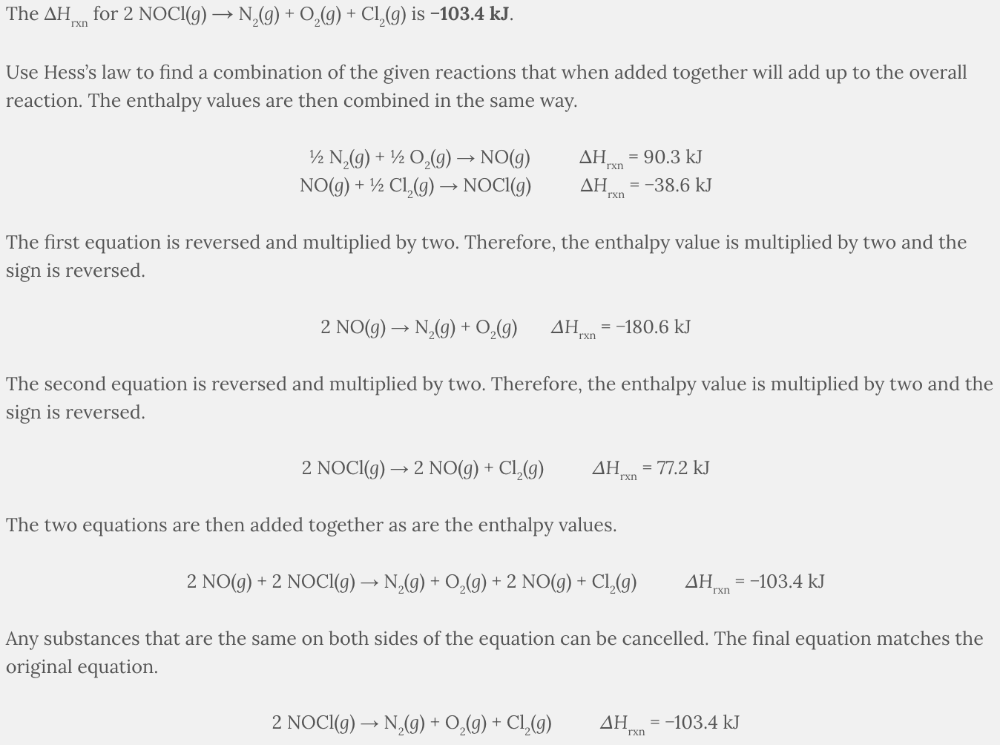
The Δ H rxn for 2 NOCl( g ) → N 2 ( g ) + O 2 ( g ) + Cl 2 ( g ) is −103.4 kJ .
Use Hess’s law to find a combination of the given reactions that when added together will add up to the overall reaction. The enthalpy values are then combined in the same way.
½ N 2 ( g ) + ½ O 2 ( g ) → NO( g ) ∆H rxn = 90.3 kJ
NO( g ) + ½ Cl 2 ( g ) → NOCl( g ) ∆H rxn = −38.6 kJ
The first equation is reversed and multiplied by two. Therefore, the enthalpy value is multiplied by two and the sign is reversed.
2 NO( g ) → N 2 ( g ) + O 2 ( g ) ∆ H rxn = −180.6 kJ
The second equation is reversed and multiplied by two. Therefore, the enthalpy value is multiplied by two and the sign is reversed.
2 NOCl( g ) → 2 NO( g ) + Cl 2 ( g ) ∆ H rxn = 77.2 kJ
The two equations are then added together as are the enthalpy values.
2 NO( g ) + 2 NOCl( g ) → N 2 ( g ) + O 2 ( g ) + 2 NO( g ) + Cl 2 ( g ) ∆ H rxn = −103.4 kJ
Any substances that are the same on both sides of the equation can be cancelled. The final equation matches the original equation.
2 NOCl( g ) → N 2 ( g ) + O 2 ( g ) + Cl 2 ( g ) ∆ H rxn = −103.4 kJ
Which substance has a standard enthalpy of formation of zero?
- Ca(s)
- F(g)
- C(diamond)
- Fe(l)
Ca(s)
Ex.
The enthalpy of formation of Ca(s) is zero.
The enthalpy of formation of an element in its standard state is zero. Ca( s ) is the standard state of calcium.
F( g ) is incorrect because the standard state of fluorine is F 2 ( g ).
Fe( l ) is incorrect because the standard state of iron is Fe( s ).
C( diamond ) is incorrect because the standard state of carbon is C( graphite ).
The specific heat capacity for a substance is the amount of heat needed to raise the temperature of 1 g of a substance by 1 °C. It is measured in units of J/(g∙°C).
specific heat capacity = J / (g∙°C)
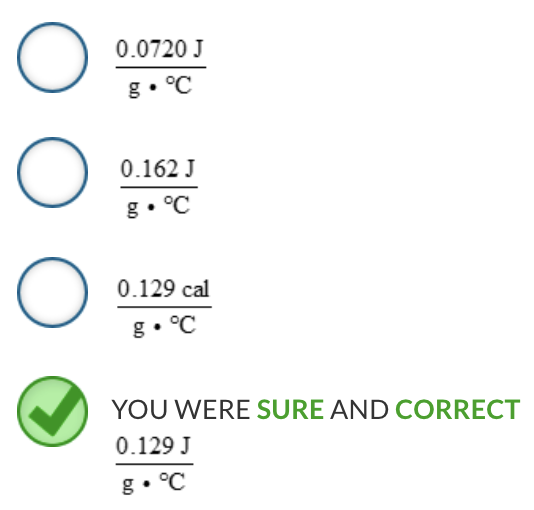
Calculate the specific heat capacity for a 15.3-g sample of gold that absorbs 87.2 J when its temperature increases from 35.0 °C to 79.2 °C.

The specific heat capacity for gold is: 0.129 J / (g∙°C)
Which statement is true ?
- The enthalpy of a reaction is the heat of reaction at constant volume.
- The enthalpy of a reaction and the enthalpy of the reverse reaction are equal.
- The enthalpy of a reaction is the negative of the enthalpy of the reverse reaction.
- The enthalpy for the formation for two moles of a substance is half of the enthalpy for the formation of one mole of the substance.
The enthalpy of a reaction is the negative of the enthalpy of the reverse reaction.
Ex.
The enthalpy of a reaction is the negative of the enthalpy of the reverse reaction.
When a reaction is reversed, the sign of the enthalpy value is changed. The energy values of the substances are the same but reversing the order changes the products to reactants and the reactants to products. When the enthalpy of formation is found, the sign is the opposite of the original reaction.
The enthalpy of a reaction is the heat of reaction at constant volume . This statement is false because enthalpy is the heat at constant pressure.
The enthalpy for the formation for two moles of a substance is half of the enthalpy for the formation of one mole of the substance . The enthalpy for the formation of two moles of a substance will be twice the enthalpy of formation for one mole. Forming more of a substance consumes or produces proportionally more energy.
The enthalpy of a reaction and the enthalpy of the reverse reaction are equal. The enthalpy of the forward and reverse reactions have equal, but opposite, signs.
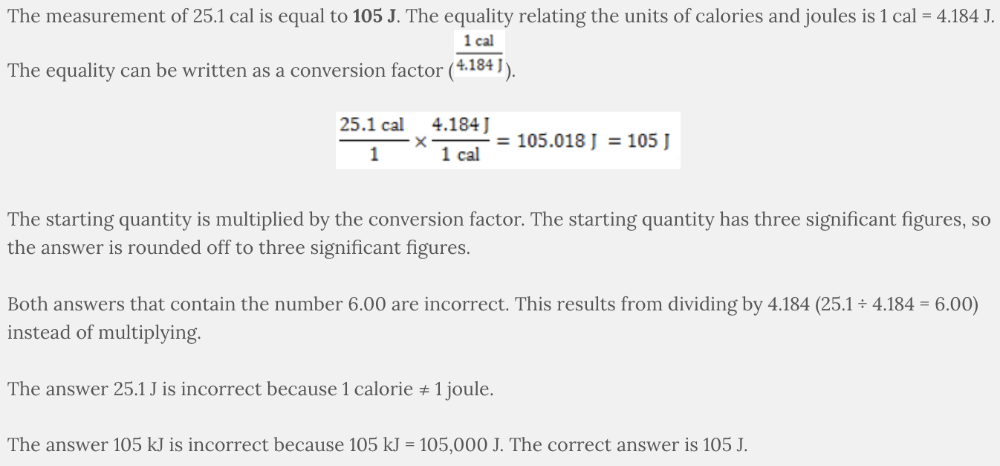
Two units of measurement for energy are calories and joules. The measurement of 25.1 cal is the same amount of energy as ___________.
- 105 kJ
- 6.00 kJ
- 6.00 J
- 25.1 J
- 105 J
105 J
Every chemical reaction will either absorb heat or release heat. A reaction that absorbs heat is an endothermic reaction, whereas a reaction that releases heat is an exothermic reaction. The release or absorption of heat is known as the heat of reaction.
Using the heat of reaction, a stoichiometry calculation can be performed to determine the amount of heat either produced or required for a reaction to occur.
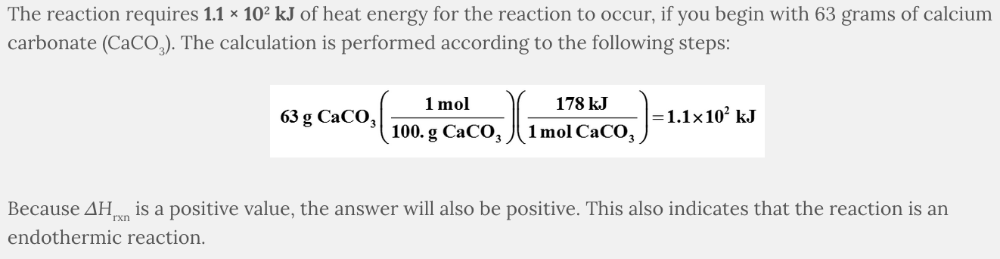
Calculate the number of kJ required for the reaction shown below, if you begin with 63 grams of calcium carbonate (CaCO 3 ), and the Δ H rxn = 178 kJ.
CaCO 3 ( s ) → CaO( s ) + CO 2 ( g )
Remember to select an answer with the correct number of significant figures.
- −56 kJ
- 1.1 × 102 kJ
- −1.1 × 102 kJ
- 56 kJ
1.1 × 10 2 kJ
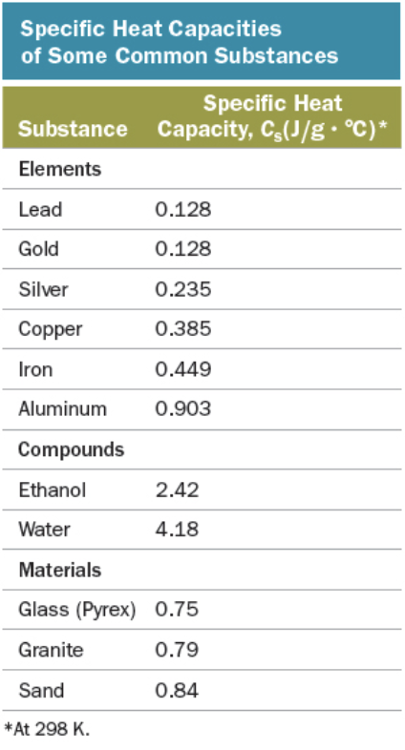
Calculate the heat required to raise the temperature of 13.5 g of ethanol from 25.8 °C to 85.6 °C.
- 10.7 J
- 334 J
- 1.95 × 103 J
- 517 J
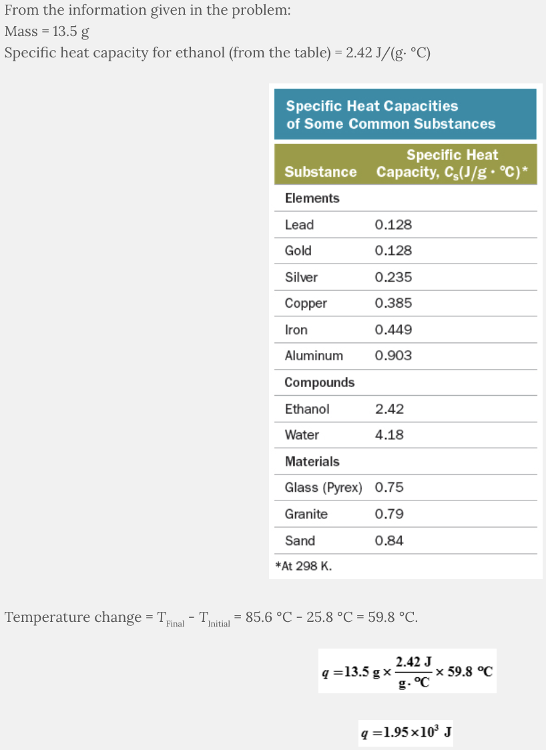
1.95 × 10 3 J
Ex.
The amount of heat absorbed by the sample of ethanol is 1.95 × 10 3 J .
The heat equation is:
q = m x Cs x ∆T
where q is heat (J), m is mass (g), C s is specific heat capacity (J/(g∙ °C)), and ΔT is change in temperature (°C).
Every chemical reaction will either absorb heat or release heat. A reaction that absorbs heat is an endothermic reaction, whereas a reaction that releases heat is an exothermic reaction. The release or absorption of heat is known as the heat of reaction.
Using the heat of reaction, a stoichiometry calculation can be performed to determine the amount of heat either produced or required for a reaction to occur.
Calculate the change in energy (in kJ) for the reaction shown below, if you begin with 75.0 grams of oxygen (O 2 ), and the Δ H rxn = −902.0 kJ.
4 NH 3 ( g ) + 5 O 2 ( g ) → 4 NO( g ) + 6 H 2 O( g )
Remember to select an answer with the correct number of significant figures.
- −77.0 kJ
- −2.11 × 103 kJ
- −902 kJ
- −423 kJ

−423 kJ
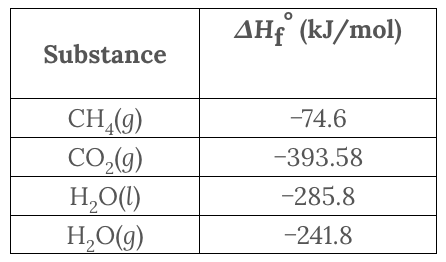
What is the standard enthalpy of the reaction CH 4 ( g ) + 2 O 2 ( g ) → CO 2 ( g ) + 2H 2 O( l )?
- −951.7 kJ
- −890.5 kJ
- −1039.7 kJ
- −802.5 kJ
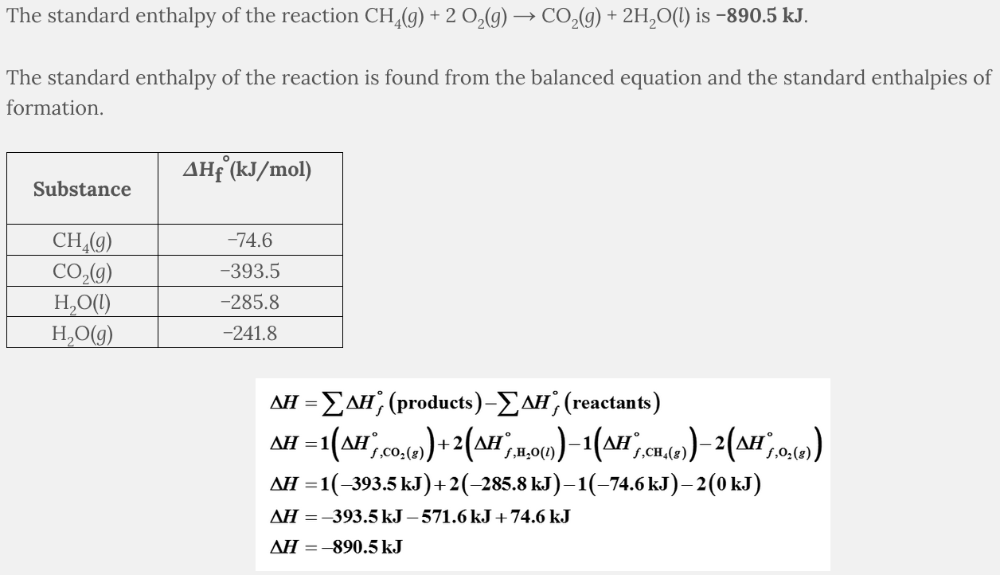
−890.5 kJ
Two units of measurement for energy are calories and joules. The measurement of 355 J is the same amount of energy as ___________.
- 355 cal
- 84.8 kcal
- 1490 kcal
- 84.8 cal
- 1490 cal
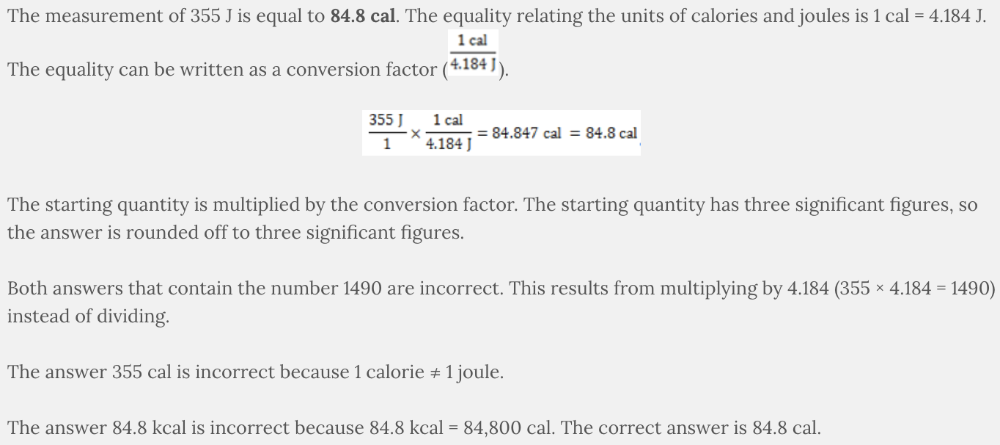
84.8 cal
The standard enthalpy of formation of liquid water is −285.8 kJ/mol. Which equation corresponds to the standard enthalpy of formation for liquid water?
- H2(g) + ½ O2(g) → H2O(l)
- 2H(g) + O(g) → H2O(l)
- 2H2(g) + O2(g) → 2H2O(l)
- H2O(g) → H2O(l)
H 2 ( g ) + ½ O 2 ( g ) → H 2 O( l )
Ex.
The equation that represents the formation of liquid water with ΔH f = −285.8 kJ/mol is H 2 (g) + ½ O 2 (g) → H 2 O(l) .
The standard enthalpy of the formation represents the formation of one mole of a compound from its elements in their standard states. H 2 ( g ) and O 2 ( g ) are the standard states of hydrogen and oxygen. Although equations are usually balanced with whole numbers, the equations for the standard enthalpy of formation must show the formation of one mole of the compound.
What is ∆H rxn for 2 CHCl 3 ( l ) → 2 C( s ) + H 2 ( g ) + 3 Cl 2 ( g ) given the standard enthalpy of formation of CHCl 3 ( l ) is −134.1 kJ/mol?
- −67.1 kJ
- −134.1 kJ
- −268.2 kJ
- 134.1 kJ
- 268.2 kJ
- 67.1 kJ

268.2 kJ
Every chemical reaction will either absorb heat or release heat. A reaction that absorbs heat is an endothermic reaction, whereas a reaction that releases heat is an exothermic reaction.
Identify the following reaction as an endothermic reaction, exothermic reaction, or both endothermic and exothermic:
Dissolving NH 4 Cl in water to make a cold pack
- Exothermic reaction
- Both endothermic and exothermic
- Endothermic reaction
Endothermic reaction
Ex.
Dissolving NH 4 Cl in water to make a cold pack is an endothermic reaction because heat is absorbed from the surroundings, resulting in a cooling effect or a decrease in temperature (i.e., a cold pack). In other words, heat is a reactant, and endothermic reactions require heat.
Endothermic reactions consume heat (heat acts as a reactant), whereas exothermic reactions produce heat (heat acts as a product).
Calculate ∆H rxn for P 4 O 6 ( s ) + 2 O 2 ( g ) → P 4 O 10 ( s ) given the following:
P 4 ( s ) + 3 O 2 ( g ) → P 4 O 6 ( s ) ∆H rxn = −1640.1 kJ
P 4 ( s ) + 5 O 2 ( g ) → P 4 O 10 ( s ) ∆H rxn = −2940.1 kJ
- 4580.2 kJ
- −4580.2 kJ
- 1300.0 kJ
- −1300.0 kJ

−1300.0 kJ
A 68-g sample of sodium is at an initial temperature of 42 °C. If 1840 joules of heat are applied to the sample, what is the final temperature of the sodium?
The specific heat capacity of sodium is 1.23 J/(g∙ °C).
- 22 °C
- 64 °C
- -20. °C
- 43 °C
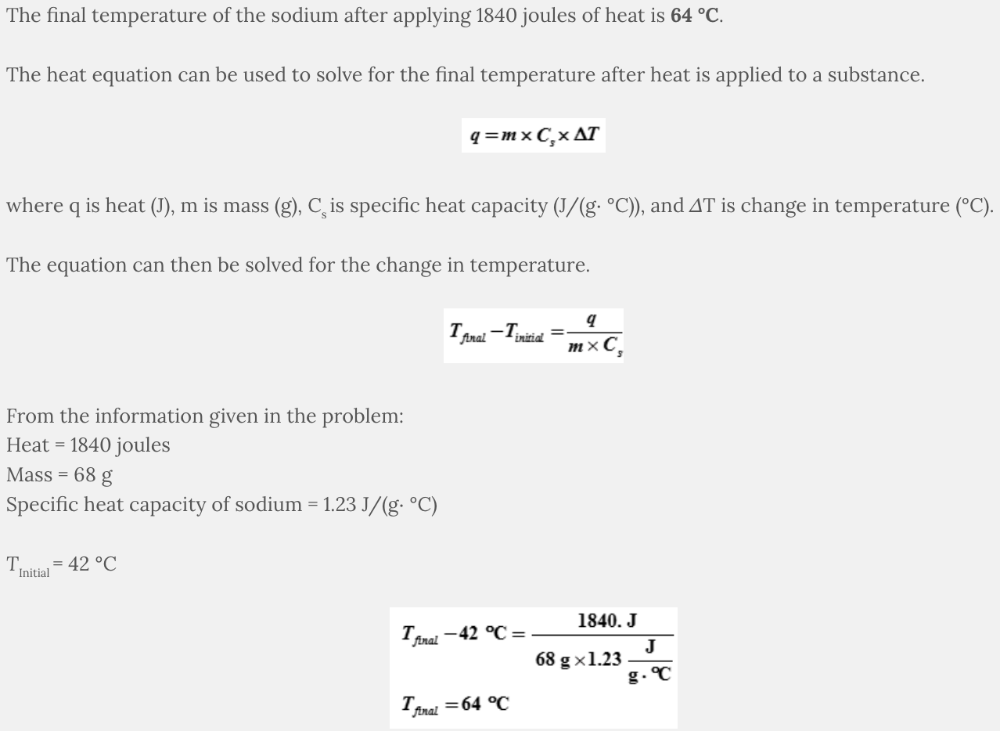
64 °C
Calculate Δ H rxn for Ca( s ) + ½ O 2 ( g ) + CO 2 ( g ) → CaCO 3 ( s ) given the following:
Ca( s ) + ½ O 2 ( g ) → CaO( s ) ∆H rxn = −635.1 kJ
CaCO 3 ( s ) → CaO( s ) + CO 2 ( g ) ∆H rxn = 178.3 kJ
- 813.4 kJ
- 456.8 kJ
- −813.4 kJ
- −456.8 kJ

−813.4 kJ
Two units of measurement for energy are calories and joules. The measurement of 8.75 kcal is the same amount of energy as ___________.
- 2.09 kJ
- 36.6 kJ
- 8.75 kJ
- 36.6 J
- 2.09 J

36.6 kJ