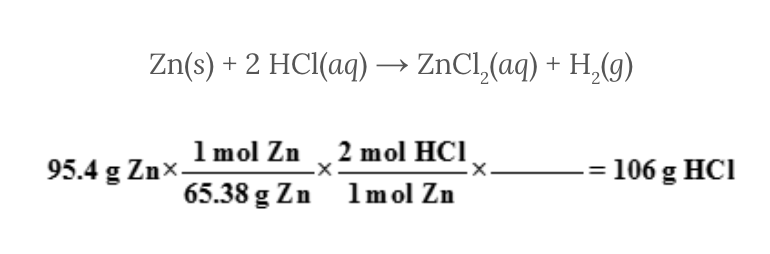
Stoichiometry is a term chemists use to describe calculations that determine the relative quantities of reactants or products involved in a chemical reaction.
Using stoichiometry, chemists can determine the amount of product that can be formed during a chemical reaction from a given amount of reactant. Chemists can also determine the amount of reactant needed to produce a desired amount of product using the same process.
Select the missing conversion factor for the following set of calculations.
Assume 95.4 grams of zinc, Zn, reacts with hydrochloric acid, HCl, to produce zinc chloride, ZnCl 2 , and hydrogen, H 2 . The problem requires that you determine the mass of hydrochloric acid, HCl, needed for the reaction to occur.
Zn( s ) + 2 HCl( aq ) → ZnCl 2 ( aq ) + H 2 ( g )

Stoichiometry is a term chemists use to describe calculations that determine the relative quantities of reactants or products involved in a chemical reaction.
Using stoichiometry, chemists can determine the amount of product that can be formed during a chemical reaction from a given amount of reactant. Chemists can also determine the amount of reactant needed to produce a desired amount of product using the same process.
What mass of AgCl will precipitate when 10.0 g of AgNO 3 is added to an aqueous solution of NaCl?
NaCl( aq ) + AgNO 3 ( aq ) → AgCl( s ) + NaNO 3 ( aq )
- 14.3 g AgCl
- 8.44 g AgCl
- 17.0 g AgCl
- 11.9 g AgCl

8.44 g AgCl
Ex.
The addition of 10.0 grams of AgNO 3 will result in the precipitation of 8.44 grams of AgCl .
Use the molar masses and the mole ratio from the coefficients of the balanced chemical equation to calculate the mass of AgCl produced.
The limiting reactant is the chemical substance that determines the amount of product(s) that can ultimately be formed in a reaction. During the reaction, the limiting reactant is completely consumed or used up, and therefore, causes the reaction to stop.
The limiting reactant can be identified through stoichiometric calculations. After comparing the results, the reactant that produces the smaller mass of product is identified as the limiting reactant.
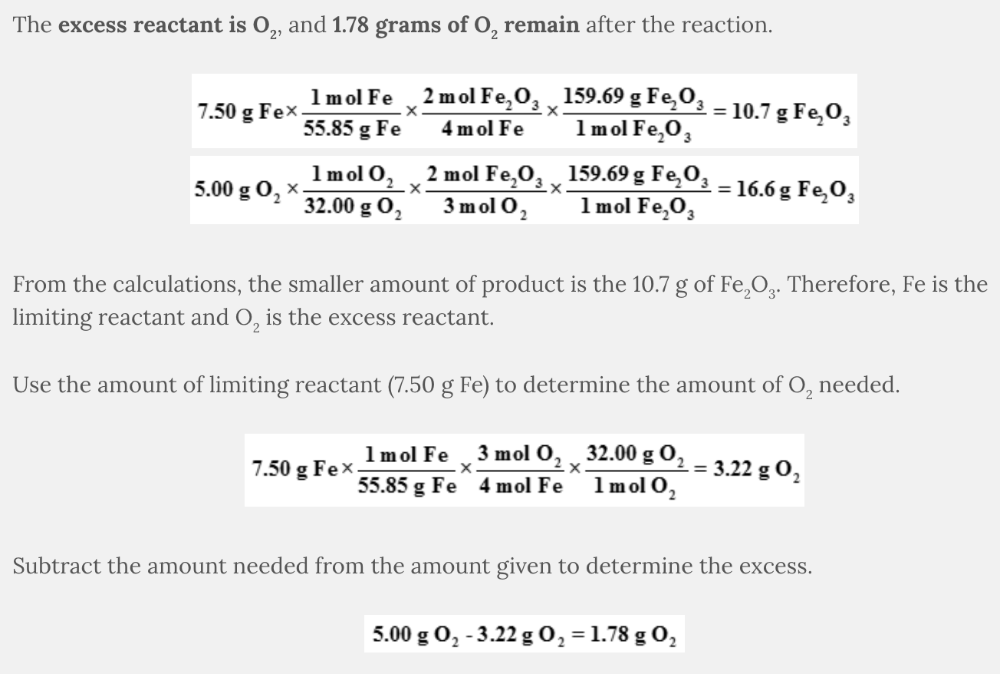
Determine the excess reactant and calculate the mass of the remaining excess reactant after 7.50 grams of Fe and 5.00 grams of O 2 react.
4 Fe( s ) + 3 O 2 ( g ) → 2 Fe 2 O 3 ( s )
- Excess reactant: O2 Grams remaining: 3.22 g of O2
- Excess reactant: O2 Grams remaining: 1.78 g of O2
- Excess reactant: Fe Grams remaining: 1.78 g of Fe
- Excess reactant: Fe Grams remaining: 3.22 g of Fe
Excess reactant: O 2
Grams remaining: 1.78 g of O 2
Ex.
The excess reactant is O 2, and 1.78 grams of O 2 remain after the reaction.
From the calculations, the smaller amount of product is the 10.7 g of Fe 2 O 3 . Therefore, Fe is the limiting reactant and O 2 is the excess reactant.
Use the amount of limiting reactant (7.50 g Fe) to determine the amount of O 2 needed.
Subtract the amount needed from the amount given to determine the excess.
A balanced chemical equation indicates the proportions or ratios of the various reactants and products to one another. From the coefficients in the balanced chemical equation, we can create mole–mole ratios that relate any of the various chemicals to a different chemical.
For example, consider the following balanced chemical equation:
N 2 ( g ) + 3 H 2 ( g ) → 2 NH 3 ( g )
From this equation, there are several possible mole–mole ratios. Two of these mole–mole ratios are the following:
3 moles H2 / 1 mole N2
OR
2 moles NH3 / 3 moles H2
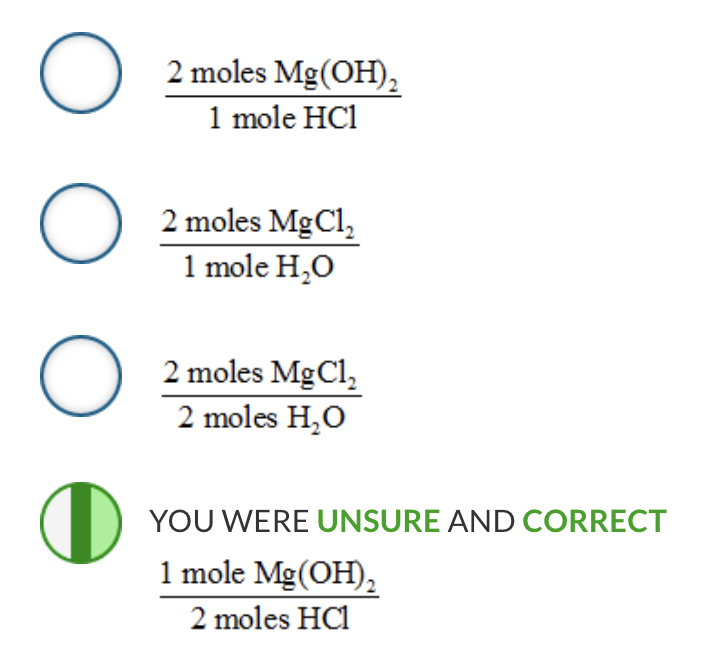
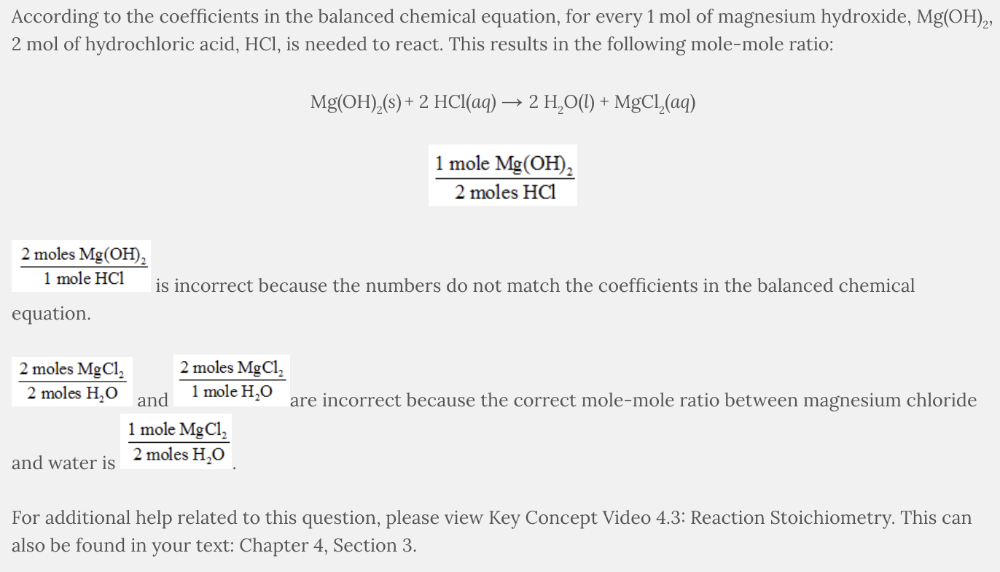
Which is a possible mole-mole ratio for the following balanced chemical equation?
Mg(OH) 2 ( s ) + 2 HCl( aq ) → 2 H 2 O( l ) + MgCl 2 ( aq )
The limiting reactant is the chemical substance that determines the amount of product(s) that can ultimately be formed in a reaction. During the reaction, the limiting reactant is completely consumed or used up and therefore causes the reaction to stop.
The limiting reactant can be identified through stoichiometric calculations. After comparing the results, the reactant that produces the smaller mass of product is identified as the limiting reactant.
You are going to have a surprise birthday party for your friend, and you are going to mail out the invitations. Each invitation needs one envelope, two stamps, one address label, two RSVP cards, and one invitation. What is your limiting reactant if you have the following materials?
7 envelopes
16 stamps
30 address labels
20 RSVP cards
10 invitations
- 16 stamps
- 30 address labels
- 10 invitations
- 7 envelopes
- 20 RSVP cards

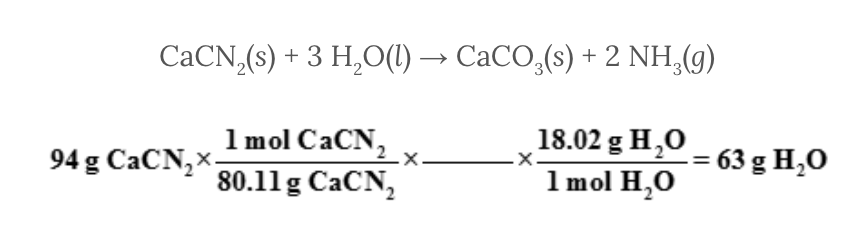
Stoichiometry is a term chemists use to describe calculations that determine the relative quantities of reactants or products involved in a chemical reaction.
Using stoichiometry, chemists can determine the amount of product that can be formed during a chemical reaction from a given amount of reactant. Chemists can also determine the amount of reactant needed to produce a desired amount of product using the same process.
Select the missing conversion factor for the following set of calculations.
Assume 94 grams of calcium cyanamide, CaCN 2 , reacts with water, H 2 O, to produce calcium carbonate, CaCO 3 , and ammonia, NH 3 . The problem requires that you determine the mass of water, H 2 O, needed for the reaction to occur.
CaCN 2 ( s ) + 3 H 2 O( l ) → CaCO 3 ( s ) + 2 NH 3 ( g )

A combustion reaction occurs when a fuel source reacts with oxygen (O 2 ) to form carbon dioxide (CO 2 ) and water (H 2 O). Combustion reactions are frequently thought of as burning a fuel source. The fuel source is typically made of carbon and hydrogen or carbon, hydrogen, and oxygen.
What is the missing equation coefficient that belongs in front of oxygen (O 2 ) in the combustion reaction for methane, CH 4 ?
CH 4 ( g ) + _ O 2 ( g ) → CO 2 ( g ) + 2 H 2 O( g )
- 4
- 3
- 1
- 2

In order to balance a reaction, the same number and type of atoms must exist on both sides of an equation. Begin by counting the number of atoms on the reactant side, and confirm that the product side has the same number of atoms. If there is a discrepancy in the number of atoms, change the equation coefficient or the number in front of the chemical formula until both sides of the equation have the same number of atoms.
What is the missing equation coefficient that belongs in front of iron (Fe) in the reaction shown below?
Fe 2 S 3 ( l ) → _ Fe( s ) + 3 S( s )
- 6
- 2
- 5
- 3

The limiting reactant is the chemical substance that determines the amount of product(s) that can ultimately be formed in a reaction. During the reaction, the limiting reactant is completely consumed or used up and therefore causes the reaction to stop.
The limiting reactant can be identified through stoichiometric calculations. After comparing the results, the reactant that produces the smaller mass of product is identified as the limiting reactant.
Determine the limiting reactant and calculate the number of grams of Al 2 O 3 that can be formed when 10.0 grams of Al and 5.00 grams of O 2 react.
4 Al( s ) + 3 O 2 ( g ) → 2 Al 2 O 3 ( s )
- Limiting reactant: 5.00 g of O2 Grams of formed: 18.9 g of Al2O3
- Limiting reactant: 10.0 g of Al Grams of formed: 10.6 g of Al2O3
- Limiting reactant: 10.0 g of Al Grams of formed: 18.9 g of Al2O3
- The Correct Answer Limiting reactant: 5.00 g of O2 Grams of formed: 10.6 g of Al2O3
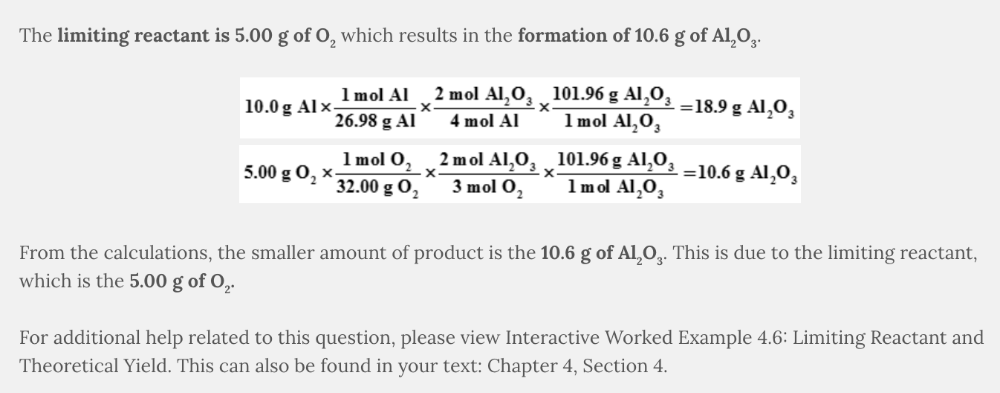
Limiting reactant: 5.00 g of O 2
Grams of formed: 10.6 g of Al 2 O 3
Stoichiometry is a term chemists use to describe calculations that determine the relative quantities of reactants or products involved in a chemical reaction.
Using stoichiometry, chemists can determine the amount of product that can be formed during a chemical reaction from a given amount of reactant. Chemists can also determine the amount of reactant needed to produce a desired amount of product using the same process.
What mass of CO 2 is produced from the decomposition of 25.5 grams of NaHCO 3 ?
NaHCO 3 ( aq ) → NaOH( aq ) + CO 2 ( g )
- 145 g CO2
- 48.7 g CO2
- 13.4 g CO2
- 3.29 g CO2
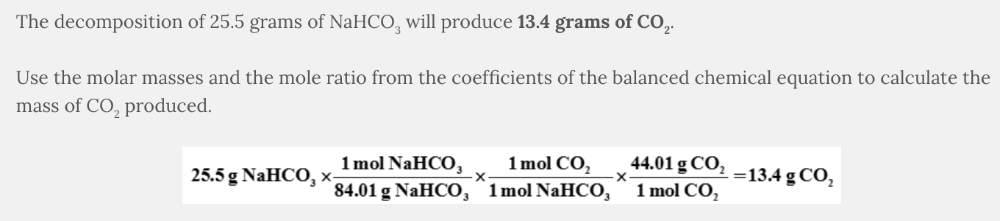
13.4 g CO 2
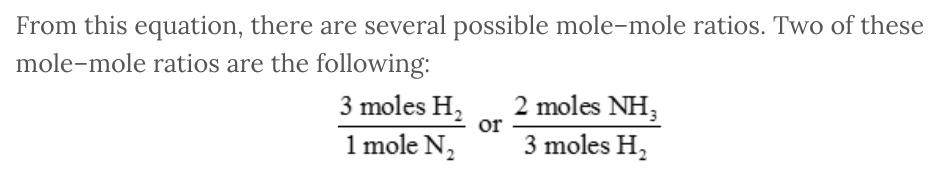
A balanced chemical equation indicates the proportions or ratios of the various reactants and products to one another. From the coefficients in the balanced chemical equation, we can create mole–mole ratios that relate any of the various chemicals to a different chemical.
For example, consider the following balanced chemical equation:
N 2 ( g ) + 3 H 2 ( g ) → 2 NH 3 ( g )
From this equation, there are several possible mole–mole ratios. Two of these mole–mole ratios are the following:
3 moles H2 / 1 mole N2
OR
2 moles NH3 / 3 moles H2
How many moles of nitrogen dioxide, NO 2 , are needed to react with 2.83 mol of water, H 2 O?
4 NO 2 ( g ) + 6 H 2 O( g ) → 7 O 2 ( g ) + 4 NH 3 ( g )
- 4.25 mol of NO2
- 1.89 mol of NO2
- 0.529 mol of NO2
- 2.83 mol of NO2

1.89 mol of NO 2

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
What is the percent yield of KOH when 22.4 grams of KO 2 react with an excess of water to produce 13.9 grams of KOH?
4 KO 2 ( s ) + 2 H 2 O( l ) → 4 KOH( s ) + 3 O 2 ( g )
- 62.1%
- 78.5%
- 79.0%
- 127%
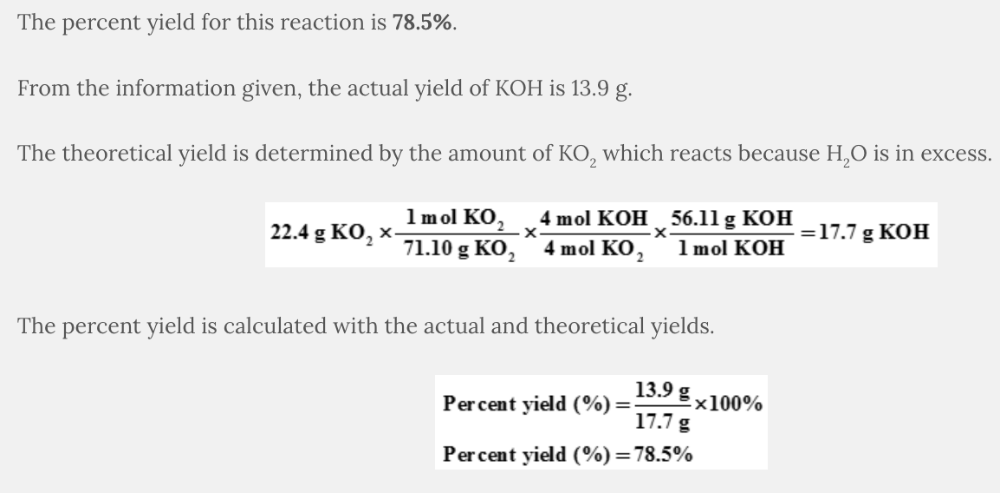
78.5%
In order to balance a reaction, the same number and type of atoms must exist on both sides of an equation. Begin by counting the number of atoms on the reactant side, and confirm that the product side has the same number of atoms. If there is a discrepancy in the number of atoms, change the equation coefficient or the number in front of the chemical formula until both sides of the equation have the same number of atoms.
After balancing the reaction, confirm that the total number of atoms is the same on both sides of the equation. In addition, confirm that all of the coefficients are in the smallest set of whole numbers.
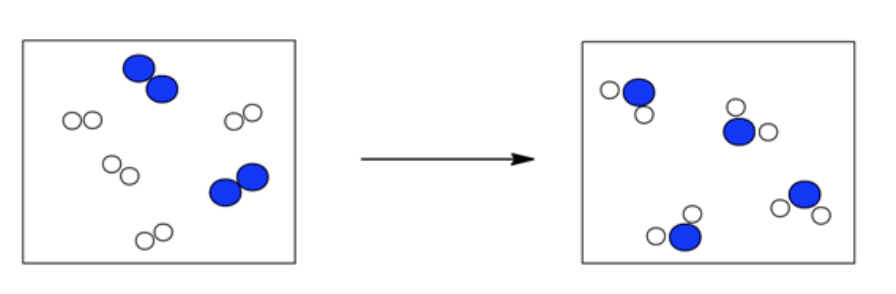
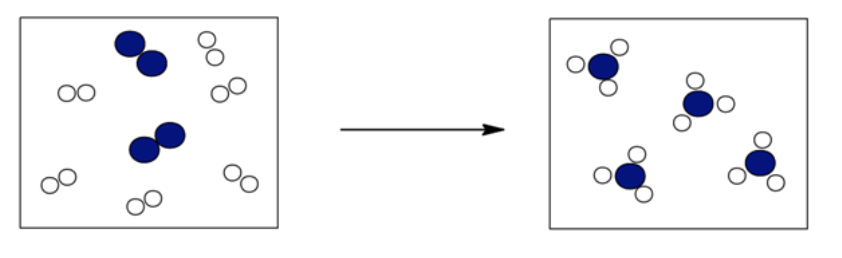
The following boxes contain hydrogen atoms (the smaller white circles) and oxygen atoms (the larger blue circles). The initial box contains H 2 ( g ) and O 2 ( g ), while the final box contains H 2 O( g ). Determine the overall reaction pictured, and write an equation that summarizes what you see. Reduce your overall equation if it is reducible. Select the balanced equation that is represented in the drawings.
- 4 O2(g) + 8 H2(g) → 4 H2O(g)
- 2 O2(g) + 4 H2(g) → 4 H2O(g)
- O2(g) + 2 H2(g) → 2 H2O(g)
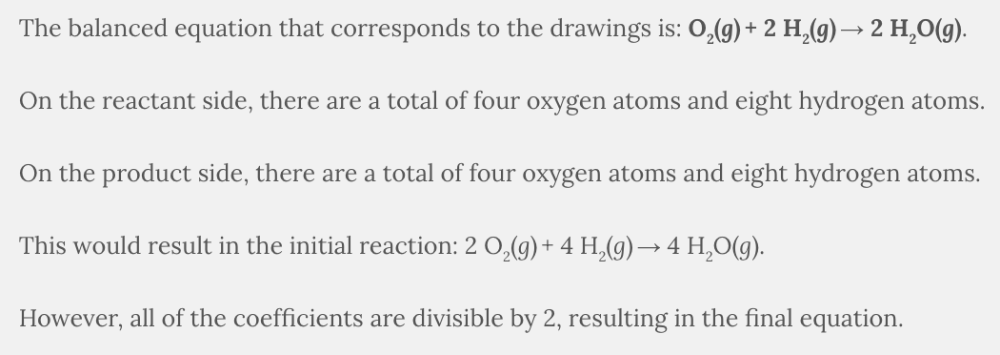
O 2 ( g ) + 2 H 2 ( g ) → 2 H 2 O( g )

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
Assume the following reaction was completed in the laboratory:
C 3 H 8 ( g ) + 5 O 2 ( g ) → 3 CO 2 ( g ) + 4 H 2 O( g )
52.2 g of propane, C 3 H 8 , reacted with 94.7 g of oxygen, O 2 , resulting in the formation of 33.0 g of carbon dioxide, CO 2 .
The following are the numerical values from the stoichiometry calculations:
- 52.2 g of propane, C 3 H 8 , formed 156 g of carbon dioxide, CO 2
- 94.7 g of oxygen, O 2 , formed 78.1 g of carbon dioxide, CO 2
What is the percent yield based on the given information?
- 237%
- 21.2%
- 55.1%
- 42.3%
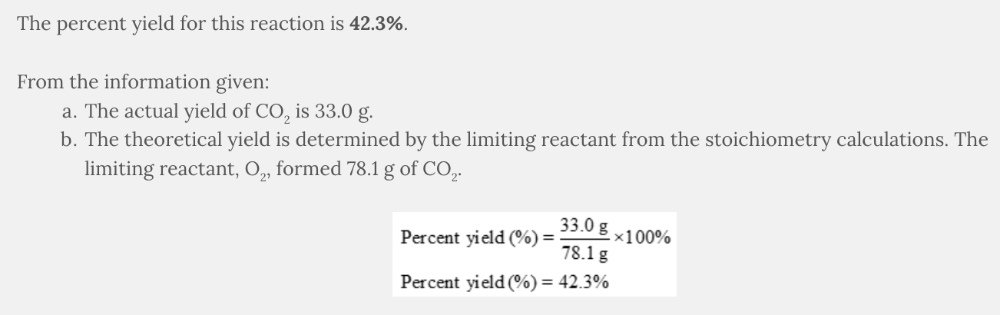
42.3%
In order to balance a reaction, the same number and type of atoms must exist on both sides of an equation. Begin by counting the number of atoms on the reactant side, and confirm that the product side has the same number of atoms. If there is a discrepancy in the number of atoms, change the equation coefficient or the number in front of the chemical formula until both sides of the equation have the same number of atoms.
What is the coefficient of boron when the following equation is balanced with small, whole-number coefficients?
_ K(s) + _ B 2 O 3(s) → _ K 2 O(s) + _ B(s)
- 2
- 3
- 4
- 1

2
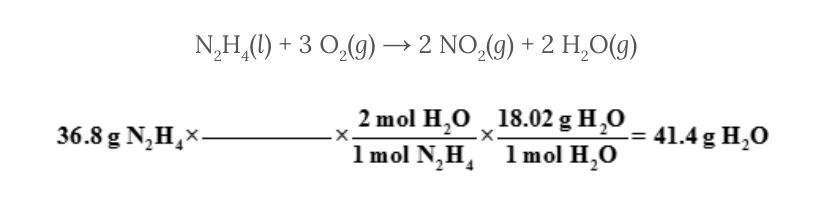
Stoichiometry is a term chemists use to describe calculations that determine the relative quantities of reactants or products involved in a chemical reaction.
Using stoichiometry, chemists can determine the amount of product that can be formed during a chemical reaction from a given amount of reactant. Chemists can also determine the amount of reactant needed to produce a desired amount of product using the same process.
Select the missing conversion factor for the following set of calculations.
Assume 36.8 grams of hydrazine, N 2 H 4 , reacts with excess oxygen, O 2 . The problem requires that you determine the mass of water, H 2 O, formed from this reaction.
N 2 H 4 ( l ) + 3 O 2 ( g ) → 2 NO 2 ( g ) + 2 H 2 O( g )
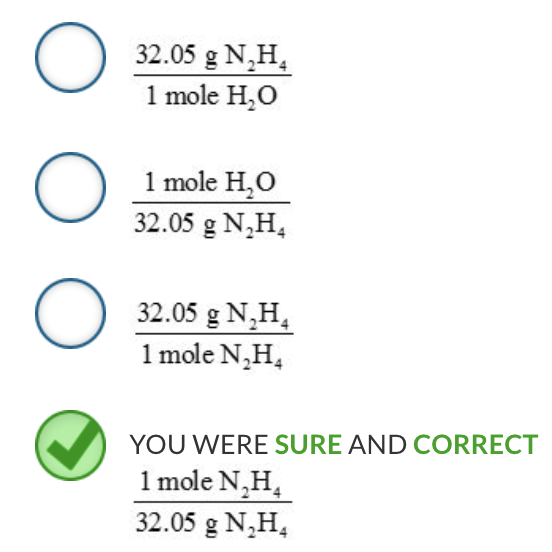
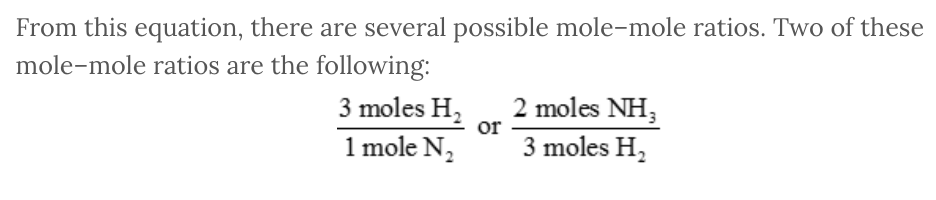
A balanced chemical equation indicates the proportions or ratios of the various reactants and products to one another. From the coefficients in the balanced chemical equation, we can create mole–mole ratios that relate any of the various chemicals to a different chemical.
For example, consider the following balanced chemical equation:
N 2 ( g ) + 3 H 2 ( g ) → 2 NH 3 ( g )
From this equation, there are several possible mole–mole ratios. Two of these mole–mole ratios are the following:
How many moles of carbon dioxide, CO 2 , are produced if 5.87 mol of glucose, C 6 H 12 O 6 , react?
C 6 H 12 O 6 ( s ) + 6 O 2 ( g ) → 6 CO 2 ( g ) + 6 H 2 O( l )
- 0.978 mol of CO2
- 35.2 mol of CO2
- 5.87 mol of CO2
- 2.84 mol of CO2
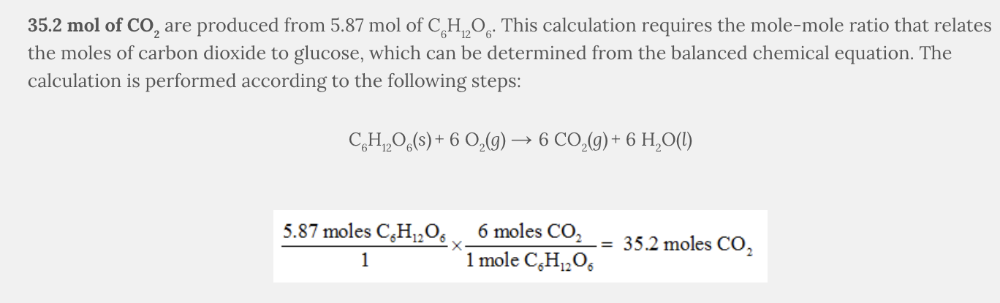
35.2 mol of CO 2

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
Assume the following reaction was completed in the laboratory:
3 Mg( s ) + N 2 ( g ) → Mg 3 N 2 ( s )
56 g of magnesium, Mg, reacted with 35 g of nitrogen, N 2 , resulting in the formation of 33 g of magnesium nitride, Mg 3 N 2 .
The following are the numerical values from the stoichiometry calculations:
- 56 g of magnesium, Mg formed 78 g of magnesium nitride, Mg 3 N 2
- 35 g of nitrogen, N 2 , formed 130 g of magnesium nitride, Mg 3 N 2
What is the percent yield based on the given information?
- 94%
- 25%
- 42%
- 236%

42%
The limiting reactant is the chemical substance that determines the amount of product(s) that can ultimately be formed in a reaction. During the reaction, the limiting reactant is completely consumed or used up, and therefore, causes the reaction to stop.
The limiting reactant can be identified through stoichiometric calculations. After comparing the results, the reactant that produces the smaller mass of product is identified as the limiting reactant.
Determine the excess reactant and calculate the mass of the remaining excess reactant after 32.5 grams of KO 2 and 27.9 grams of H 2 O react.
4 KO 2 ( s ) + 2 H 2 O( l ) → 4 KOH( s ) +3 O 2 ( g )
- Excess reactant: KO2 Grams remaining: 16.5 g of KO2
- Excess reactant: H2O Grams remaining: 16.5 g of H2O
- Excess reactant: H2O Grams remaining: 23.8 g of H2O
- Excess reactant: KO2 Grams remaining: 11.4 g of KO2

Excess reactant: H 2 O
Grams remaining: 23.8 g of H 2 O

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
What is the percent yield of ZnCl 2 when 19.2 grams of Zn react with an excess of HCl to produce 28.2 grams of ZnCl 2 ?
Zn( s ) + 2 HCl( aq ) → ZnCl 2 (aq) + H 2 ( g )
- 48.0%
- 68.1%
- 142%
- 70.5%
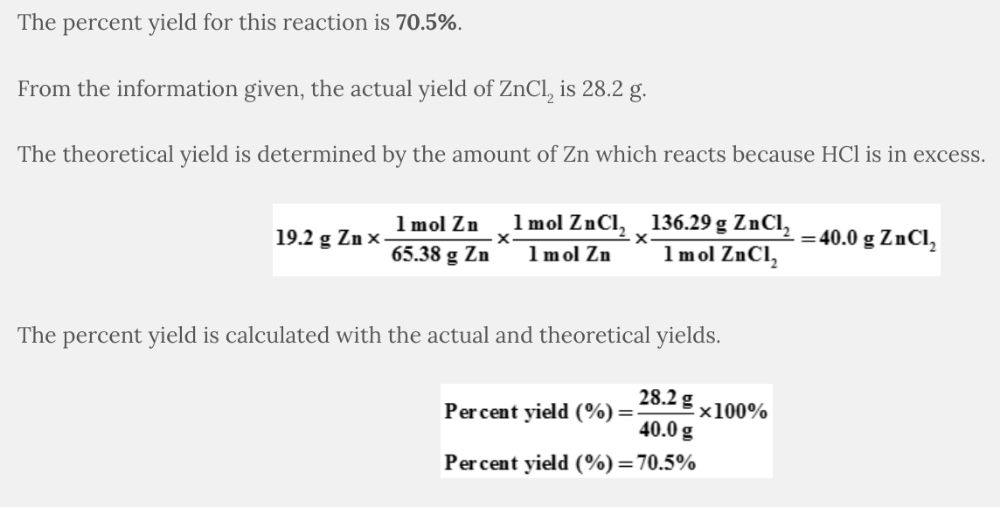
70.5%

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
Aluminum oxide (Al 2 O 3 ) is produced according to the following equation.
4 Al( s ) + 3 O 2 ( g ) → 2 Al 2 O 3 ( s )
If the reaction occurs with an 82.4% yield, what mass of aluminum should be reacted with excess oxygen to produce 45.0 grams of Al 2 O 3 ?
- 37.9 g Al
- 35.1 g Al
- 28.9 g Al
- 23.8 g Al
- 54.6 g Al
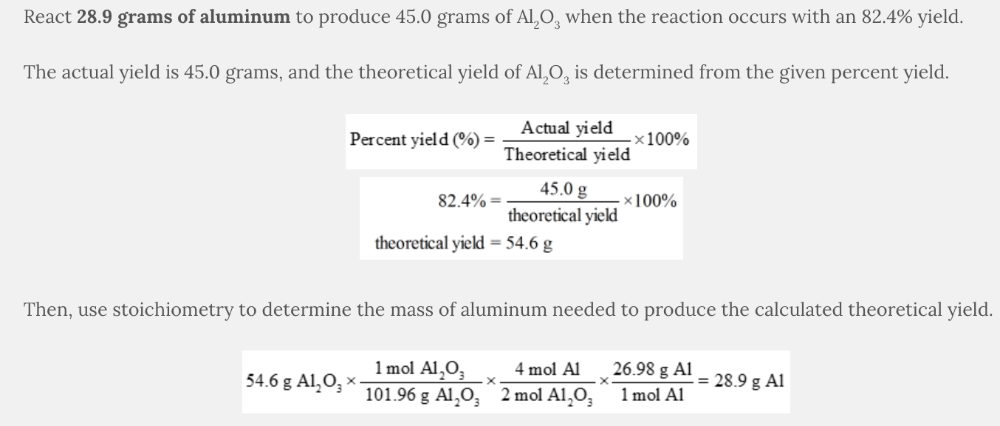
28.9 g Al
Stoichiometry is a term chemists use to describe calculations that determine the relative quantities of reactants or products involved in a chemical reaction.
Using stoichiometry, chemists can determine the amount of product that can be formed during a chemical reaction from a given amount of reactant. Chemists can also determine the amount of reactant needed to produce a desired amount of product using the same process.
A sample of 35.0 g of magnesium undergoes oxidation. What mass of MgO is produced?
2 Mg( s ) + O 2 ( g ) → 2 MgO( s )
- 116 g MgO
- 29.0 g MgO
- 21.1 g MgO
- 58.0 g MgO
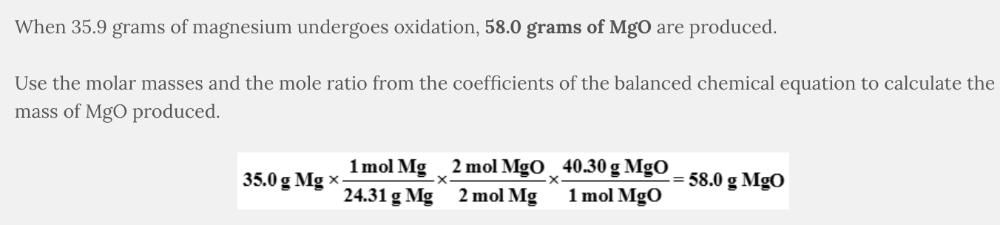
58.0 g MgO
In order to balance a reaction, the same number and type of atoms must exist on both sides of an equation. Begin by counting the number of atoms on the reactant side, and confirm that the product side has the same number of atoms. If there is a discrepancy in the number of atoms, change the equation coefficient or the number in front of the chemical formula until both sides of the equation have the same number of atoms.
What is the coefficient of KCl when the following equation is balanced with small, whole-number coefficients?
_ KClO 3 (s) → _ KCl (s) + _ O(g)
- 2
- 4
- 1
- 3

2
The limiting reactant is the chemical substance that determines the amount of product(s) that can ultimately be formed in a reaction. During the reaction, the limiting reactant is completely consumed or used up and therefore causes the reaction to stop.
The limiting reactant can be identified through stoichiometric calculations. After comparing the results, the reactant that produces the smaller mass of product is identified as the limiting reactant.
Determine the limiting reactant and calculate the number of grams of carbon dioxide, CO 2 that can be formed when 125 g of iron(III) oxide, Fe 2 O 3 , reacts with 196 g of carbon monoxide, CO.
Fe 2 O 3 ( s ) + 3 CO( s ) → 2 Fe( s ) + 3 CO 2 ( g )
- Limiting reactant: 125 g of Fe2O3 Grams of carbon dioxide formed: 34.4 g of CO2
- Limiting reactant: 196 g of CO Grams of carbon dioxide formed: 308 g of CO2
- Limiting reactant: 196 g of CO Grams of carbon dioxide formed: 103 g of CO2
- Limiting reactant: 125 g of Fe2O3 Grams of carbon dioxide formed: 103 g of CO2
- Limiting reactant: 125 g of Fe2O3 Grams of carbon dioxide formed: 34.4 g of CO2
- Limiting reactant: 196 g of CO Grams of carbon dioxide formed: 308 g of CO2
- Limiting reactant: 196 g of CO Grams of carbon dioxide formed: 103 g of CO2
- Limiting reactant: 125 g of Fe2O3 Grams of carbon dioxide formed: 103 g of CO2
Limiting reactant: 125 g of Fe 2 O 3
Grams of carbon dioxide formed: 103 g of CO 2
Stoichiometry is a term chemists use to describe calculations that determine the relative quantities of reactants or products involved in a chemical reaction.
Using stoichiometry, chemists can determine the amount of product that can be formed during a chemical reaction from a given amount of reactant. Chemists can also determine the amount of reactant needed to produce a desired amount of product using the same process.
What mass of CO 2 is produced from the combustion of 65.3 grams of CH 4 ?
CH 4 ( g ) + 2 O 2 ( g ) → CO 2 ( g ) + 2 H 2 O( l )
- 179 g CO2
- 23.8 g CO2
- 461 g CO2
- 108 g CO2
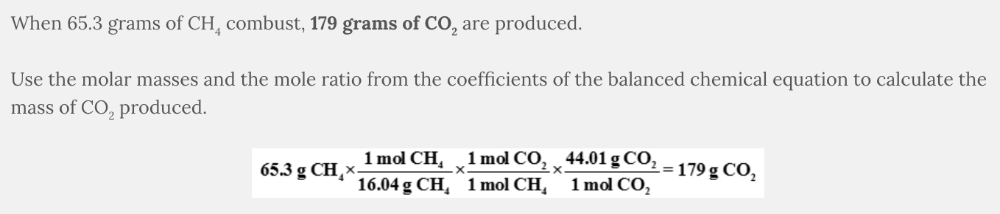
179 g CO 2
The limiting reactant is the chemical substance that determines the amount of product(s) that can ultimately be formed in a reaction. During the reaction, the limiting reactant is completely consumed or used up, and therefore, causes the reaction to stop.
The limiting reactant can be identified through stoichiometric calculations. After comparing the results, the reactant that produces the smaller mass of product is identified as the limiting reactant.
Determine the excess reactant and calculate the mass of the remaining excess reactant after 12.5 grams of Zn and 14.2 grams of HCl react.
Zn( s ) + 2 HCl( aq ) → ZnCl 2 (aq) + H 2 ( g )
- Excess reactant: Zn Grams remaining: 0.3 g of Zn
- Excess reactant: HCl Grams remaining: 13.9 g of HCl
- Excess reactant: Zn Grams remaining: 13.9 g of Zn
- Excess reactant: HCl Grams remaining: 0.3 g of HCl

Excess reactant: HCl
Grams remaining: 0.3 g of HCl

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
What is the percent yield of NH 3 when 15.0 grams of nitrogen gas react with an excess of hydrogen gas to produce 15.4 grams of NH 3 ?
N 2 ( g ) + 3 H 2 ( g ) → 2 NH 3 ( g )
- 118%
- 84.6%
- 82.4%
- 97.4%
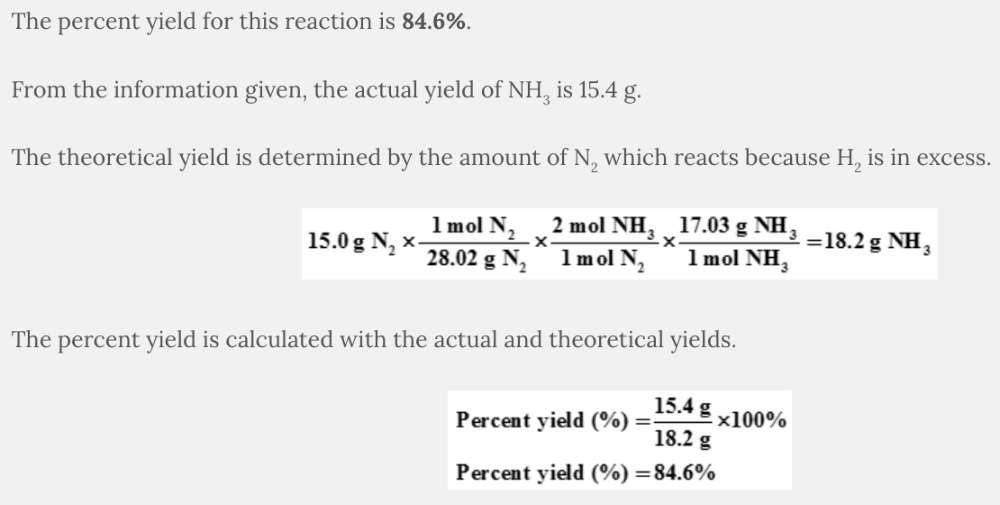
84.6%
In order to balance a reaction, the same number and type of atoms must exist on both sides of an equation. Begin by counting the number of atoms on the reactant side, and confirm that the product side has the same number of atoms. If there is a discrepancy in the number of atoms, change the equation coefficient or the number in front of the chemical formula until both sides of the equation have the same number of atoms.
What is the coefficient of aluminum when the following equation is balanced with small, whole-number coefficients?
_ Al(s)+ _ HCl(g) → _ AlCl 3(s) + _ H 2(g)
- 1
- 6
- 2
- 3

2
In order to balance a reaction, the same number and type of atoms must exist on both sides of an equation. Begin by counting the number of atoms on the reactant side, and confirm that the product side has the same number of atoms. If there is a discrepancy in the number of atoms, change the equation coefficient or the number in front of the chemical formula until both sides of the equation have the same number of atoms.
After balancing the reaction, confirm that the total number of atoms is the same on both sides of the equation. In addition, confirm that all of the coefficients are the smallest set of whole numbers.
The following boxes contain fluorine atoms (the smaller white circles) as well as nitrogen atoms (the larger blue circles). The initial box contains F 2 ( g ) and N 2 ( g ), while the final box contains NF 3 ( g ). Determine the overall reaction pictured, and write an equation that summarizes what you see. Reduce your overall equation if it is reducible. Select the balanced equation that is represented in the drawings.
- 2 N2(g) + 6 F2(g) → 4 NF3(g)
- 4 N2(g) + 12 F2(g) → 4 NF3(g)
- N2(g) + 3 F2(g) → 2 NF3(g)
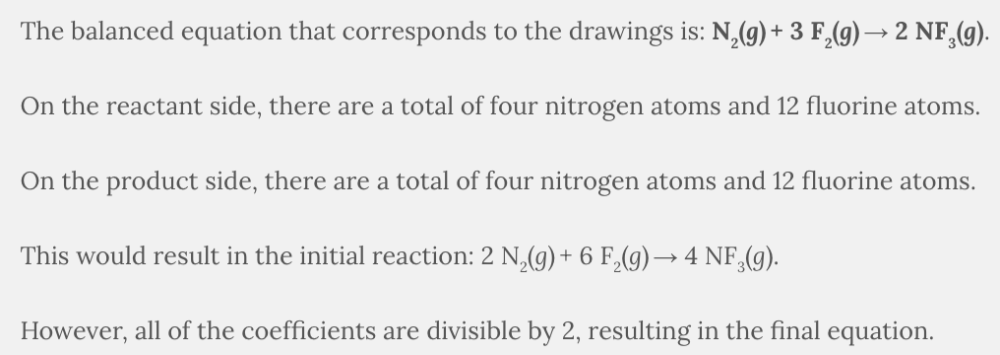
N 2 ( g ) + 3 F 2 ( g ) → 2 NF 3 ( g )

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
Assume the following reaction was completed in the laboratory:
6 Na( s ) + N 2 ( g ) → 2 Na 3 N( s )
75 g of sodium, Na, reacted with 45 g of nitrogen, N 2 , resulting in the formation of 68 g of sodium nitride, Na 3 N.
The following are the numerical values from the stoichiometry calculations:
- 75 g of sodium, Na, formed 90. g of sodium nitride, Na 3 N
- 45 g of nitrogen, N 2 , formed 260 g of sodium nitride, Na 3 N
What is the percent yield based on the given information?
- 91%
- 25%
- 132%
- 76%
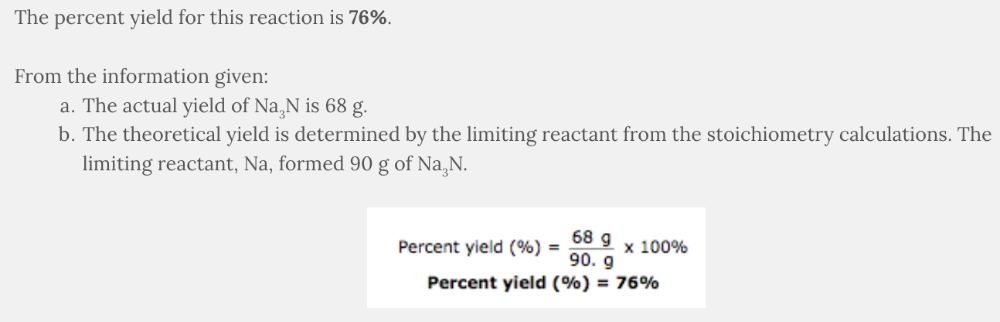
76%
Stoichiometry is a term chemists use to describe calculations that determine the relative quantities of reactants or products involved in a chemical reaction.
Using stoichiometry, chemists can determine the amount of product that can be formed during a chemical reaction from a given amount of reactant. Chemists can also determine the amount of reactant needed to produce a desired amount of product using the same process.
What mass of AgCl will precipitate when 10.0 g of NaCl is added to an aqueous solution of AgNO 3 ?
NaCl( aq ) + AgNO 3 ( aq ) → AgCl( s ) + NaNO 3 ( aq )
- 5.84 g AgCl
- 14.3 g AgCl
- 24.5 g AgCl
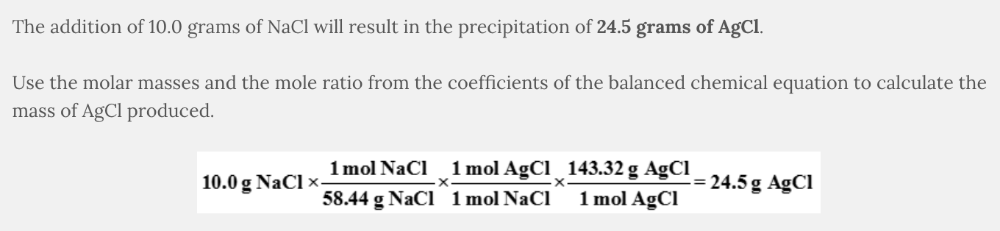
24.5 g AgCl
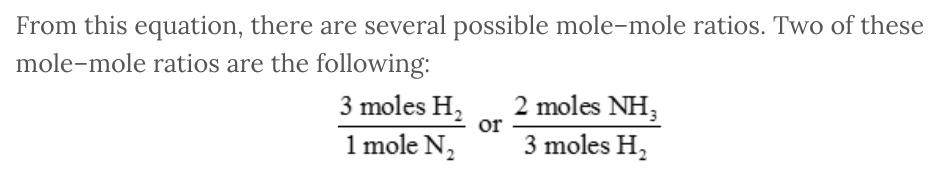
A balanced chemical equation indicates the proportions or ratios of the various reactants and products to one another. From the coefficients in the balanced chemical equation, we can create mole–mole ratios that relate any of the various chemicals to a different chemical.
For example, consider the following balanced chemical equation:
N 2 ( g ) + 3 H 2 ( g ) → 2 NH 3 ( g )
From this equation, there are several possible mole–mole ratios. Two of these mole–mole ratios are the following:
How many moles of aluminum, Al, are needed to react with 4.7 mol of chlorine, Cl 2 ?
2 Al( s ) + 3 Cl 2 ( g ) → 2 AlCl 3 ( s )
- 3.1 mol of Al
- 7.1 mol of Al
- 4.7 mol of Al
- 2.7 mol of Al
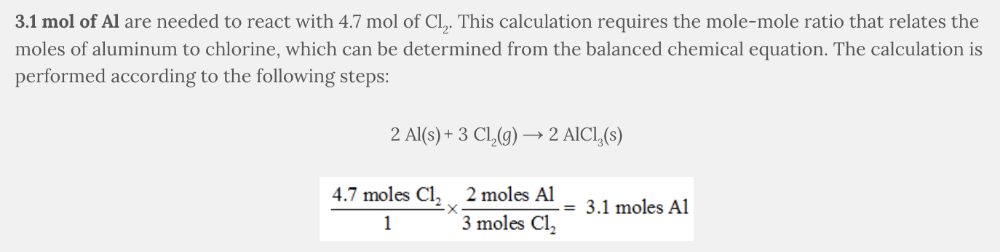
3.1 mol of Al

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
Iron(III) oxide (Fe 2 O 3 ) is produced according to the following equation:
4 Fe( s ) + 3 O 2 ( g ) 2 Fe 2 O 3 ( s )
If the reaction occurs with an 73.9% yield, what mass of iron should be reacted to produce 63.0 grams of Fe 2 O 3
- 44.1 g Fe
- 32.6 g Fe
- 80.8 g Fe
- 85.3 g Fe
- 59.7 g Fe
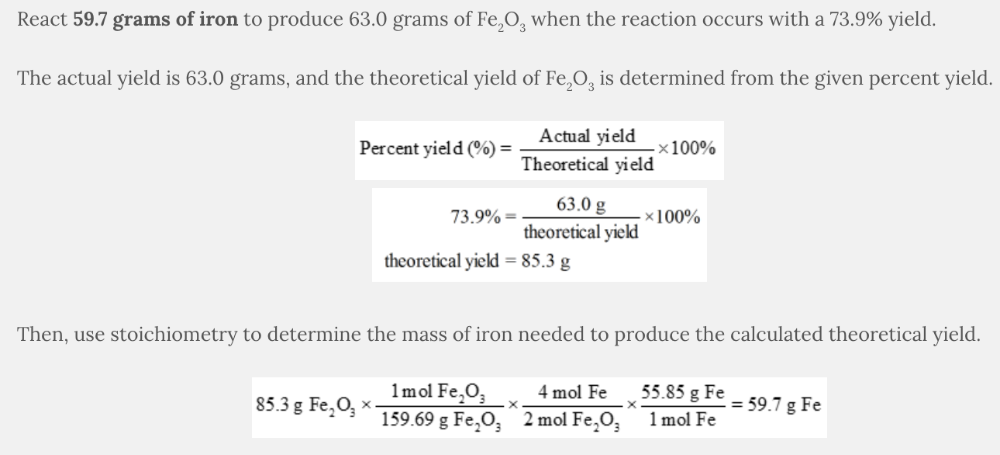
59.7 g Fe

Percent yield is a numerical value that indicates the success of a reaction in terms of how much product was actually made.
If all of the reactants were completely converted to products, the percent yield would be 100%. However, this rarely occurs due to loss of product during transfers, incomplete reactions, etc.
The percent yield is calculated as follows:
The theoretical yield is the mass of product theoretically created as determined by the stoichiometry calculations.
The actual yield is the mass of product actually created in the laboratory when the experiment is performed.
Assume the following reaction was completed in the laboratory:
4 Fe( s ) + 3 O 2 ( g ) → 2 Fe 2 O 3 ( s )
83 g of iron, Fe, reacted with 39 g of oxygen, O 2 , resulting in the formation of 98 g of iron(III) oxide, Fe 2 O 3 .
The following are the numerical values from the stoichiometry calculations:
- 83 g of iron, Fe, formed 119 g of iron(III) oxide, Fe 2 O 3
- 39 g of oxygen, O 2 , formed 130. g of iron(III) oxide, Fe 2 O 3
What is the percent yield based on the given information?
- 121%
- 85%
- 77%
- 82%
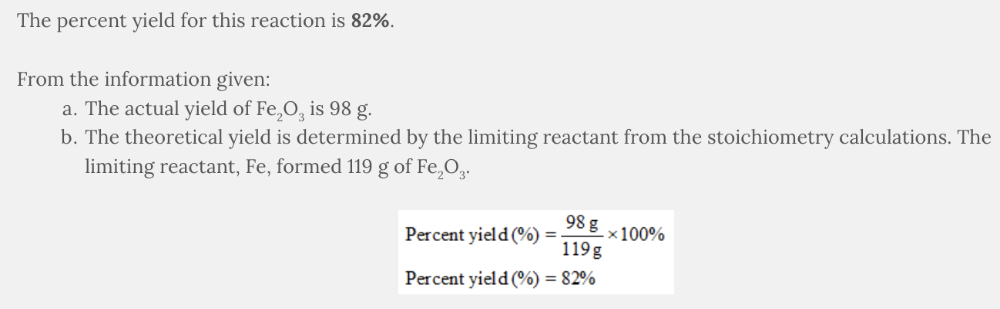
82%
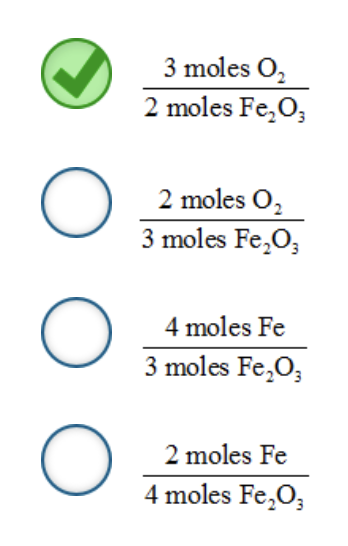
A balanced chemical equation indicates the proportions or ratios of the various reactants and products to one another. From the coefficients in the balanced chemical equation, we can create mole–mole ratios that relate any of the various chemicals to a different chemical.
For example, consider the following balanced chemical equation:
N 2 ( g ) + 3 H 2 ( g ) → 2 NH 3 ( g )
From this equation, there are several possible mole–mole ratios. Two of these mole–mole ratios are the following:
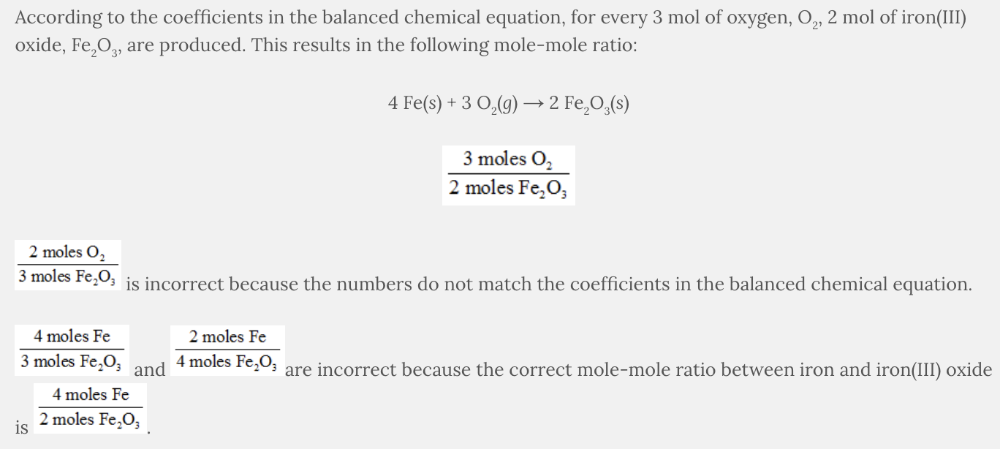
Which is a possible mole–mole ratio for the following balanced chemical equation?
4 Fe( s ) + 3 O 2 ( g ) → 2 Fe 2 O 3 ( s )