Hydrogen bonding is a special case of dipole-dipole interaction.
True
Temporary dipoles are mainly responsible for Van Der Waal bonding interactions.
True
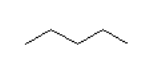
What is the main intermolecular force with the following compound?
Zigzag with 4 lines.
- Van der Waal
- Dipole-dipole
- Hydrogen bonding
Van der Waal
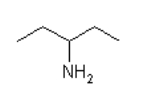
What is the main intermolecular force with the following compound?
Five line zigzag with N H2 off third point
Zigzag with 4 lines and N H2 coming off 3rd point.
- Van der Waal
- Dipole-dipole
- Hydrogen bonding
Hydrogen bonding
Ex.
OH, NH, FH
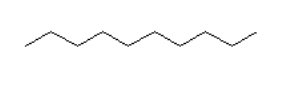
What is the main intermolecular force with the following compound?
Zigzag with 8 lines.
- Van der Waal
- Dipole-dipole
- Hydrogen bonding
Van der Waal
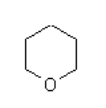
What is the main intermolecular force with the following compound?
Hexagon with O at one of the vertices.
- Van der Waal
- Dipole-dipole
- Hydrogen bonding
Dipole-dipole
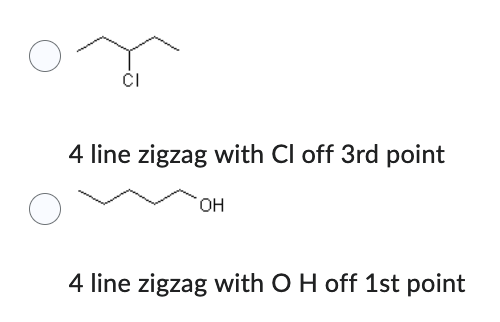
Which compound has the lower solubility in water?
- 4 line zigzag with Cl off 3rd point
- 4 line zigzag with O H off 1st point
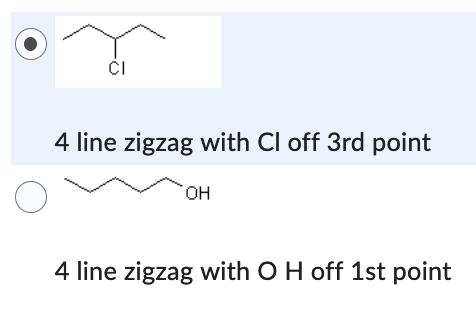
4 line zigzag with Cl off 3rd point
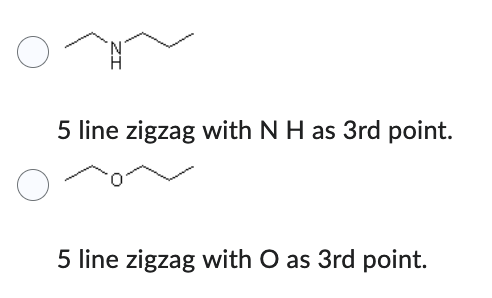
Which compound has the lower solubility in hexane (C6H14) ?
- 5 line zigzag with N H as 3rd point.
- 5 line zigzag with O as 3rd point.
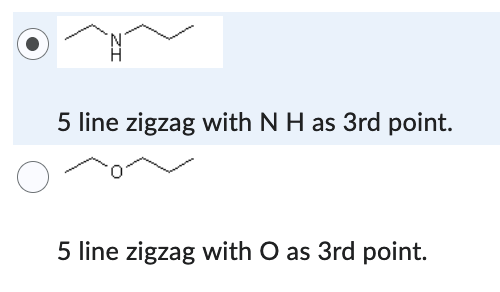
5 line zigzag with N H as 3rd point.
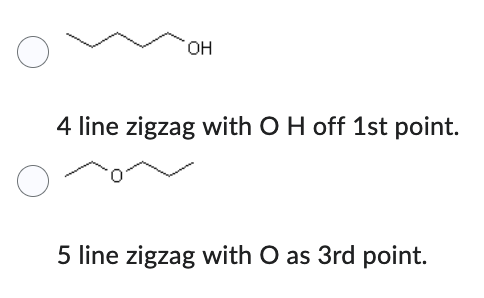
Which compound has the lower solubility in water?
- 4 line zigzag with O H off 1st point.
- 5 line zigzag with O as 3rd point.
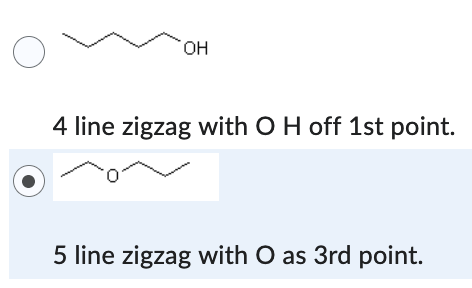
5 line zigzag with O as 3rd point.
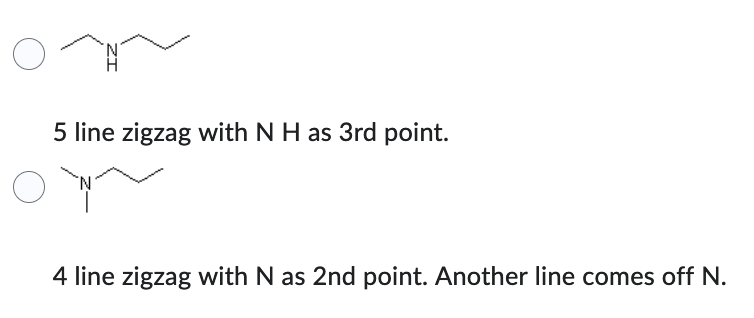
Which compound has the higher solubility in hexane (C6H14) ?
- 5 line zigzag with N H as 3rd point.
- 4 line zigzag with N as 2nd point. Another line comes off N.

4 line zigzag with N as 2nd point. Another line comes off N.
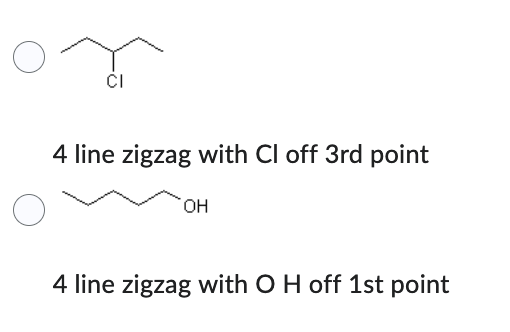
Which compound has the higher solubility in hexane (C6H14) ?
- 4 line zigzag with Cl off 3rd point
- 4 line zigzag with O H off 1st point
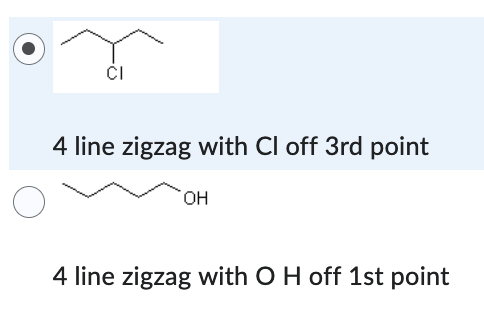
4 line zigzag with with Cl off 3rd point
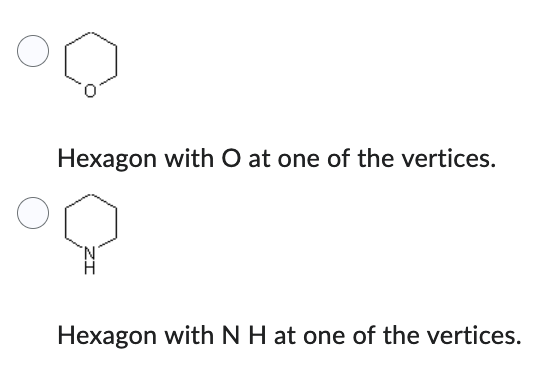
Which compound has the lower boiling point?
- Hexagon with O at one of the vertices.
- Hexagon with N H at one of the vertices.
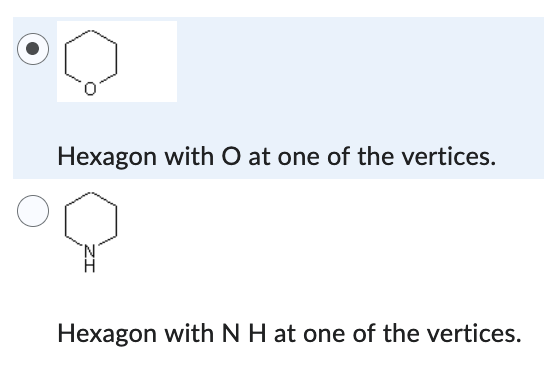
Hexagon with O at one of the vertices.
Which compound has the lower boiling point?
- 5 line zigzag with O as 3rd point.
- 5 line zigzag with N H as 3rd point.
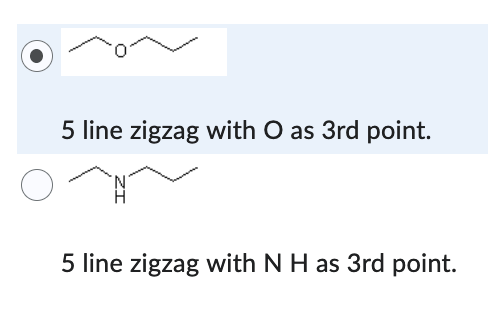
5 line zigzag with O as 3rd point.
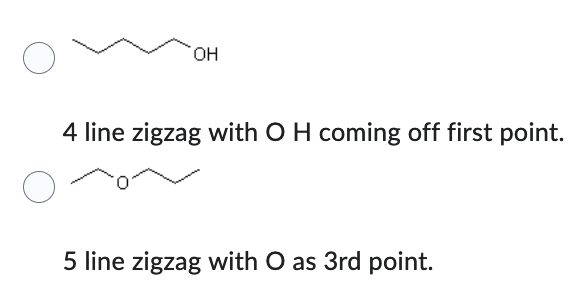
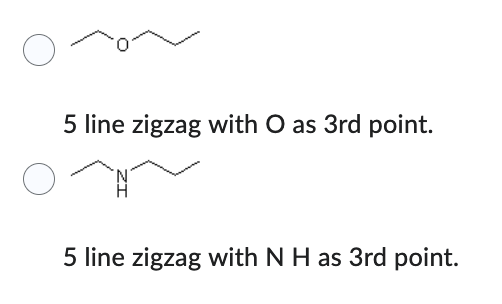
Which compound has the lower solubility in hexane (C6H14) ?
- 4 line zigzag with O H coming off first point.
- 5 line zigzag with O as 3rd point.
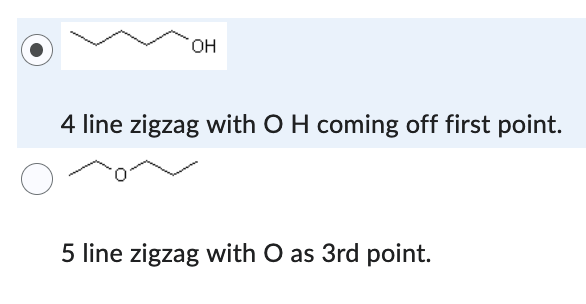
4 line zigzag with O H coming off first point.
An unknown liquid has a heat of vaporization of 36.45 kJ/mole. If the normal boiling point is 81, what is vapor pressure (in torr) of this liquid at room temperature of 25 degrees C? HINT: Normal boiling point occurs when the vapor pressure of the liquid is the same as atmospheric pressure (1 atm or 760 mm Hg).
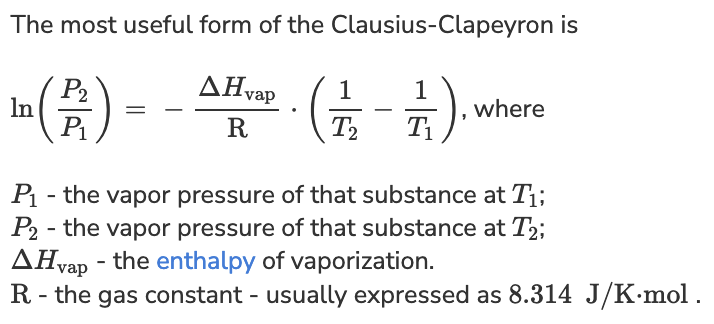
P1 = 760 torr
P2 = ?
T1 = 81 degrees C + 273 = 354 K
T2 = 25 degrees C + 273 = 298 K
delta H = 36.45 kJ/mole x (1000 J / 1 kJ) = 36,450 J/mole
R = 8.314 K/J/mole
ln (P2 / P1) = - (delta H / R) x (1/T2 - 1/T1)
P2 = P1 e[-(delta H / R) x (1/T2 - 1/T1)]
P2 = 760 torr e[-( 36,450 J/mole / 8.314 K/J/mole) x (1/298 K - 1/354 K)]
P2 = 74 torr
Ex.
In a calculator, input:
(760)e^((-36450/8.314)(1/298-1/354))
How much heat is required to heat 10.34 grams of a solid from a temperature of -14.14°C to a gas at 127°C? If the process is exothermic be sure to include the proper sign with your answer.
Assume the molar mass of the sample is 21.99 grams/mole
Melting point: 4°C
Boiling point: 80°C
Specific heat of the solid: 1.67 J/g°C
Specific heat of liquid: 4.29 J/g°C
Specific heat of gas: 1.65 J/g°C
Enthalpy of fusion: 5.21 kJ/mol
Enthalpy of vaporization: 30.95 kJ/mol
Report your answer in kJ
Do not type units with your answer.
Moles of substance = mass / molar mass = 10.34 g / 21.99 g/mol = 0.470214 mol
Q1 (solid, ice) = 10.34 g x 1.67 J/g°C x (4°C-(-14.14°C)) = 313.238 J x (1 kJ / 1000 J) = 0.313238 kJ
Q2 (fusion, ice and water) = 5.21 kJ/mol x 0.470214 mol = 2.44981494 kJ
Q3 (liquid, water) = 10.34 g x 4.29 J/g°C x (80°C-4°C) = 3371.2536 J x (1 kJ / 1000 J) = 3.37125 kJ
Q4 (vaporization, water and steam) = 30.95 kJ/mol x 0.470214 mol = 14.5531233 kJ
Q5 (gas, steam) = 10.34 g x 1.65 J/g°C x (127°C-80°C) = 801.867 J x (1 kJ / 1000 J) = 0.801867 kJ
delta E = 0.313238 + 2.44981494 + 3.37125 + 14.5531233 + 0.801867 = 21.5 kJ
How much heat is required to heat 11.87 grams of a liquid from a temperature of 19.8°C to a gas at 122.4°C? If the process is exothermic be sure to include the proper sign with your answer.
Assume the molar mass of the sample is 29.05 grams/mole
Melting point: 4.6°C
Boiling point: 61.2°C
Specific heat of the solid: 1.83 J/g°C
Specific heat of liquid: 1.97 J/g°C
Specific heat of gas: 1.73 J/g°C
Enthalpy of fusion: 2.62 kJ/mol
Enthalpy of vaporization: 32.24 kJ/mol
Report your answer in kJ
Do not type units with your answer.
Moles of substance = mass / molar mass = 11.87 g / 29.05 g/mol = 0.408606 mol
Q1 (solid, ice) = Not needed
Q2 (fusion, ice and water) = Not needed
Q3 (liquid, water) = 11.87 g x 1.97 J/g°C x (61.2°C-4.6°C) = 1323.52874 J x (1 kJ / 1000 J) = 1.32353 kJ
Q4 (vaporization, water and steam) = 32.24 kJ/mol x 0.408606 mol = 13.17345744 kJ
Q5 (gas, steam) = 11.87 g x 1.73 J/g°C x (122.4°C-61.2°C) = 1256.74812 J x (1 kJ / 1000 J) = 1.25675 kJ
delta E = 1.32353 + 13.17345744 + 1.25675 = 15.8 kJ
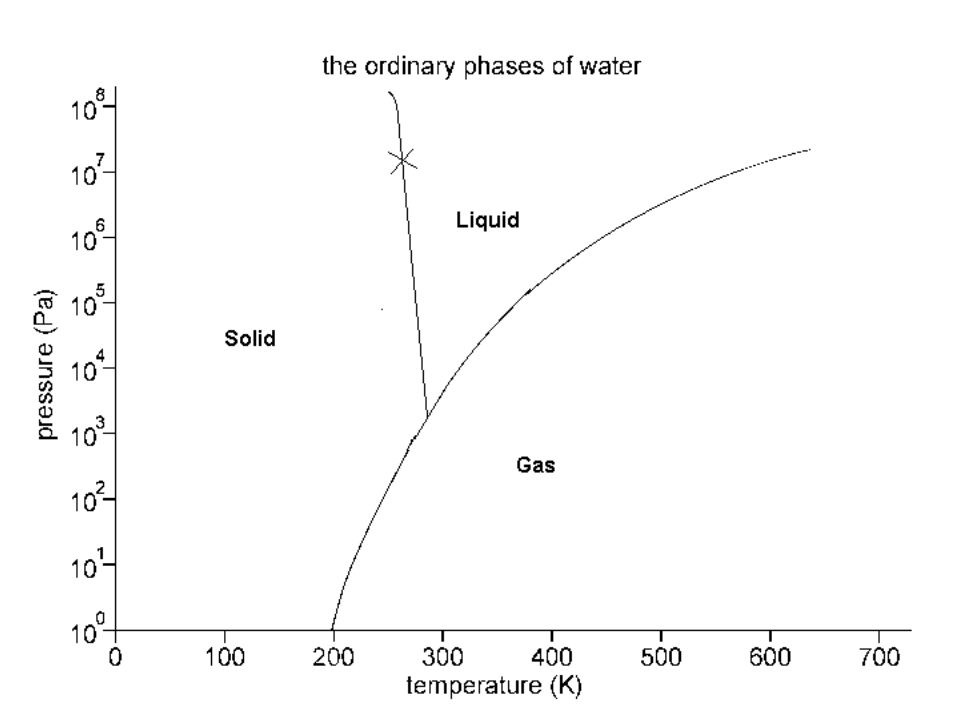
Consider the phase diagram below:
The critical temperature is:
Screen Reader Description: The Ordinary Phases of Water. Image: Graph of Temp (Kelvins from 0 to 700) on X axis, P (atm) on Y axis (from 10 to the power of 0 to 10 to the power of 8, pressure, (P a)). There is a curve starting at 200 on the X axis and 10 to the power of 0 on the Y axis. The line goes upward to the right to finish at 610 on the X axis and 10 to the power of 7 on the Y axis. There is a straight line with a slightly negative slope starting at 260 on the X axis and 10 to the power of 8 on the Y axis that goes downward until it hits the curve and stops. To the left of the straight line is the word "Solid". Between the straight line on the right and the curve is the word "Liquid". To the right side of the curve is the word "Gas". End of image.
700 K
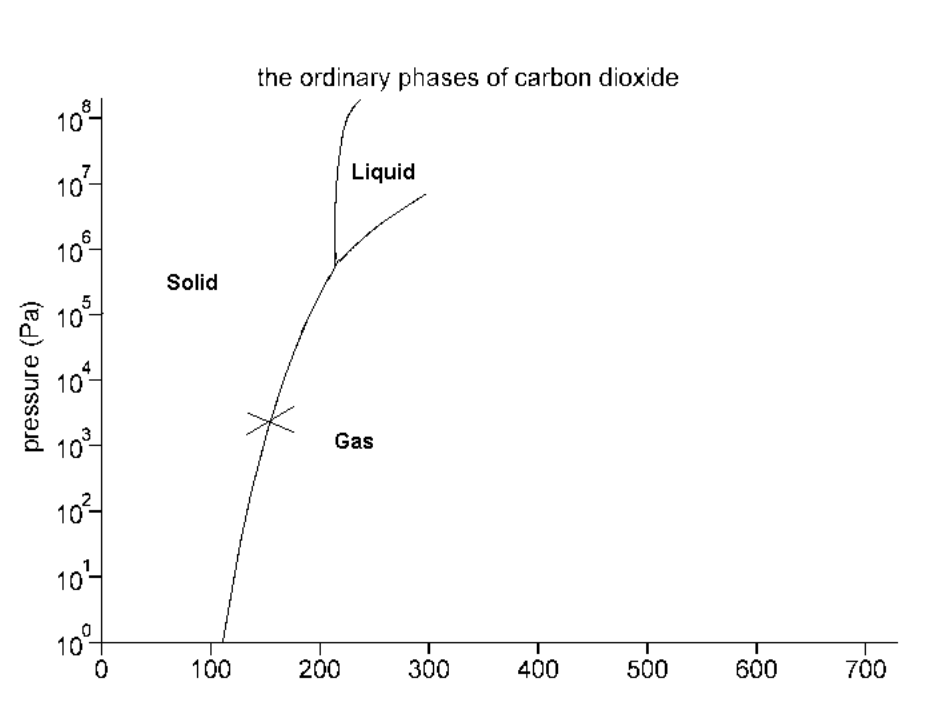
Consider the phase diagram below:
What phase(s) is/are present at point X?
Screen reader description: The Ordinary Phases of Carbon Dioxide. Pressure (Pa) 10 to the power of 0 to 10 up to 10 to the power of 8 on Y-axis. Temperature in Kelvins 0 to 700 on X-axis. Curved line up from 105 K at 10 to the power of 0 Pa up to 200 K and 10 to the power of 6 Pa. The line forks here. There is an almost vertical line up to 10 to the power of 8 Pa, and a line that goes diagonally up and to the right to 325 K and 10 to the power of 7 Pa. To the left of the fork is "Solid". To the right of the fork is "Gas". In between the lines of the fork is "Liquid". There is an X on the first curved line at 150, 10 to the power of 3. End of image.
Solid and Gas
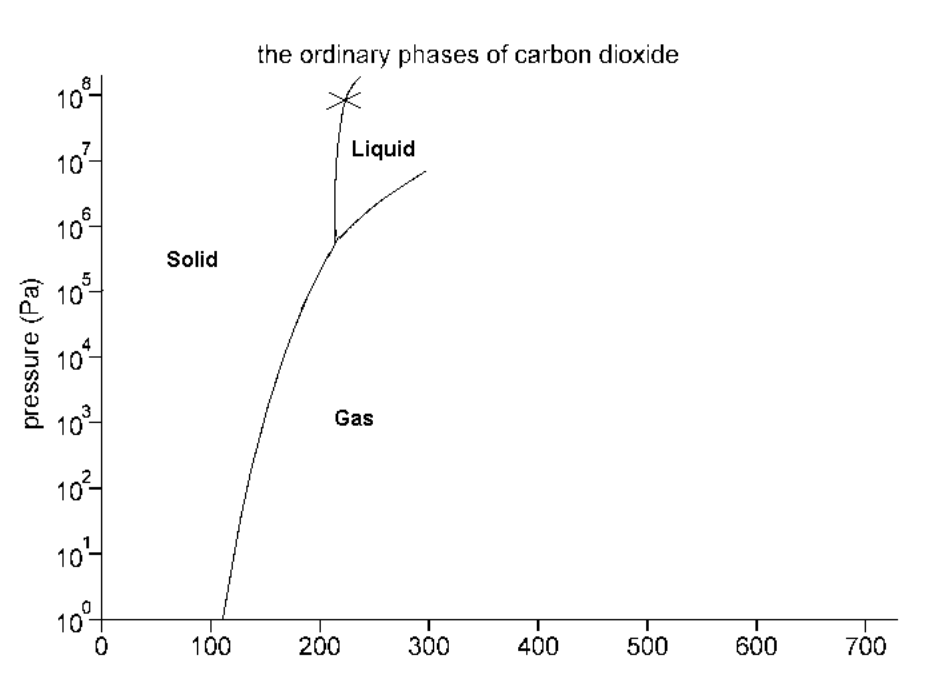
Consider the phase diagram below:
What phase(s) is/are present at point X?
Screen reader description: The Ordinary Phases of Carbon Dioxide. Pressure (Pa) 10 to the power of 0 to 10 up to 10 to the power of 8 on Y-axis. Temperature in Kelvins 0 to 700 on X-axis. Curved line up from 105 K at 10 to the power of 0 Pa up to 200 K and 10 to the power of 6 Pa. The line forks here. There is an almost vertical line up to 10 to the power of 8 Pa, and a line that goes diagonally up and to the right to 325 K and 10 to the power of 7 Pa. To the left of the fork is "Solid". To the right of the fork is "Gas". In between the lines of the fork is "Liquid". There is an X on the left fork at 200 K and 10 to the power of 8. End of image.
Solid and Liquid
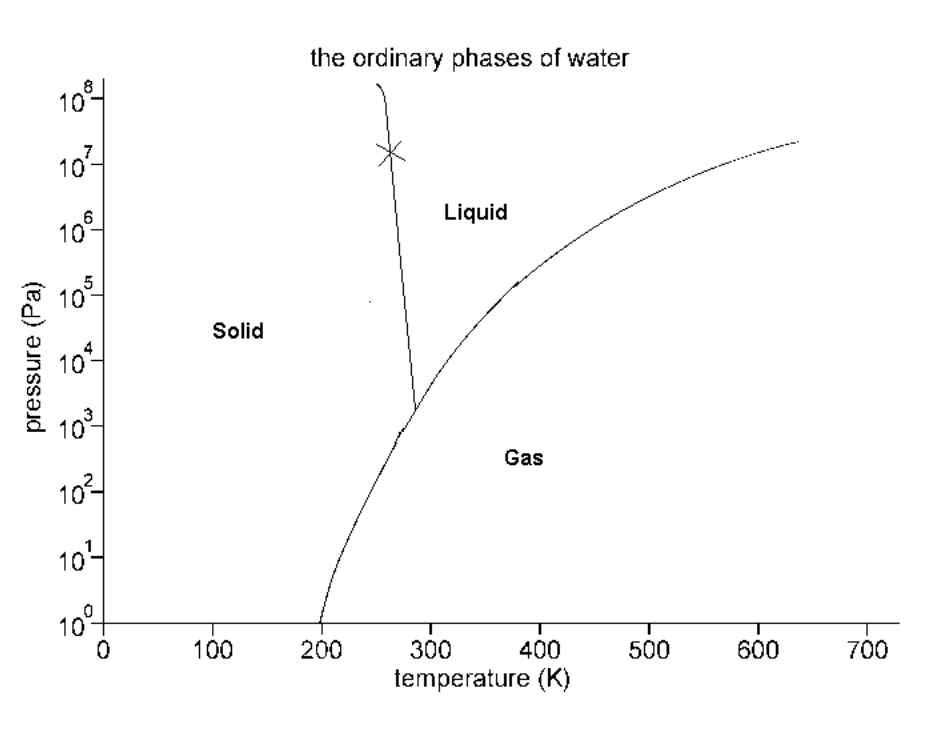
Consider the phase diagram below:
What phase(s) is/are present at 10000 Pa and 200 K?
Screen Reader Description: The Ordinary Phases of Water. Image: Graph of Temp (Kelvins from 0 to 700) on X axis, P (atm) on Y axis (from 10 to the power of 0 to 10 to the power of 8, pressure, (P a)). There is a curve starting at 200 on the X axis and 10 to the power of 0 on the Y axis. The line goes upward to the right to finish at 610 on the X axis and 10 to the power of 7 on the Y axis. There is a straight line with a slightly negative slope starting at 260 on the X axis and 10 to the power of 8 on the Y axis that goes downward until it hits the curve and stops. To the left of the straight line is the word "Solid". Between the straight line on the right and the curve is the word "Liquid". To the right side of the curve is the word "Gas". End of image.
Solid