Consider the following compound: NI2 - (OR N I2 1 minus)
What is the Electron Domain Geometry?
Tetrahedral
Consider the following compound: S i Br3 - (S i Br3 1 minus)
What is the Electron Domain Geometry?
Tetrahedral
Consider the following compound: K r I2
What is the Electron Domain Geometry?
Trigonal bipyramidal
Consider the following compound: S i F3 - (S i F3 1 minus)
What is the Electron Domain Geometry?
Tetrahedral
Consider the following compound: SbCl3 2- (OR Sb Cl3 2 minus)
What is the Molecular Geometry?
T-shaped
Consider the following compound: GaF3 2- (OR G a F3 2 minus)
What is the Molecular Geometry?
Trigonal pyramidal
Consider the following compound: S i H3 - (OR S i H3 1 minus)
What is the Molecular Geometry?
Trigonal pyramidal
Consider the following compound: C F3 - (OR C F3 1 minus)
What is the Molecular Geometry?
Trigonal pyramidal
What is the hybridization of the central atom in A sBr5?
sp3d
What is the hybridization of the central atom in NF3 2- (OR N F3 2 minus)?
sp3d
What is the hybridization of the central atom in KrF2?
sp3d
What is the hybridization of the central atom in N Cl5?
sp3d
What is the bond angle of the central atom in S i I3 - (OR S i I3 1 minus)?
109.5 o
What is the bond angle of the central atom in CS3 2- (OR C S3 2 minus)?
120 o
What is the bond angle of the central atom in S I4?
120 o/ 90 o
What is the bond angle of the central atom in SbF2 - (OR Sb F2 1 minus)?
109.5 o
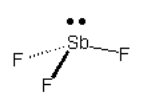
Consider the compound below:
Screen Reader Note: Sb with lone pair on top. One F atom connected to Sb in the plane, down to the right. One F atom going into the page down and to the left. One F atom coming out of the page, down and to the left. End of Note.
Which arrow depicted below is the most accurate representation of the net dipole for this compound?
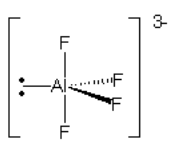
Consider the compound below:
Screen Reader Note: Al with lone pair in plane pointing left. One F straight up in plane. One F straight down in plane. One F into page pointing right. One F out of page pointing right. End of note.
Which arrow depicted below is the most accurate representation of the net dipole for this compound?
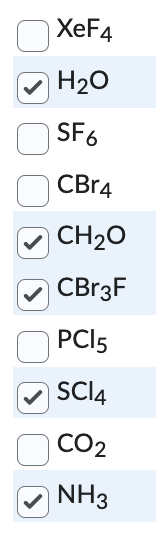
Which of the following molecules do you expect to be polar? You may need to draw the molecular geometries of each of the molecules to make this determination.
Select all correct answers.
Draw the Lewis Structure of C2H4.
C2H4 Contains how many total sigma bonds? (type a number (1,2,4, etc.)
______
Draw the Lewis Structure of C2H4.
C2H4 Contains how many total pi bonds? (type a number (1,2,4, etc.)
______
sigma = ___5___
pi = ___1___
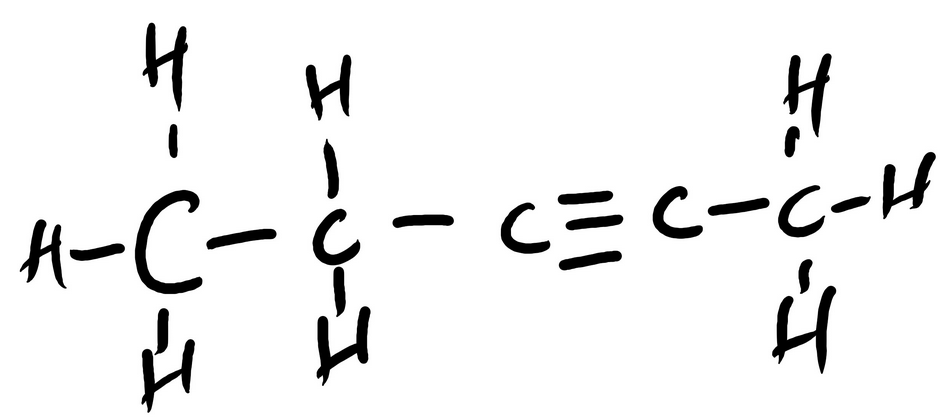
CH3CH2CCCH3 Contains how many total sigma bonds? (type a number (1,2,4, etc.)
______
CH3CH2CCCH3 Contains how many total pi bonds? (type a number (1,2,4, etc.)
______
sigma = ___12___
pi = ___2___
How many electrons are in anti-bonding orbitals in B2?
Be sure to use all the electrons when constructing your MO diagram. This includes the core and valence electrons.
4
How many electrons are in anti-bonding orbitals in O2?Be sure to use all the electrons when constructing your MO diagram. This includes the core and valence electrons.
6
What is the bond order of O2?
2
What is the bond order of B e2?
0
Draw a molecular orbital diagram for N2 -1. Is this molecule paramagnetic or diamagnetic?
- paramagnetic
- diamagnetic
paramagnetic