Consider the following compound: CI3 - (C I3 1 minus)
What is the Electron Domain Geometry?
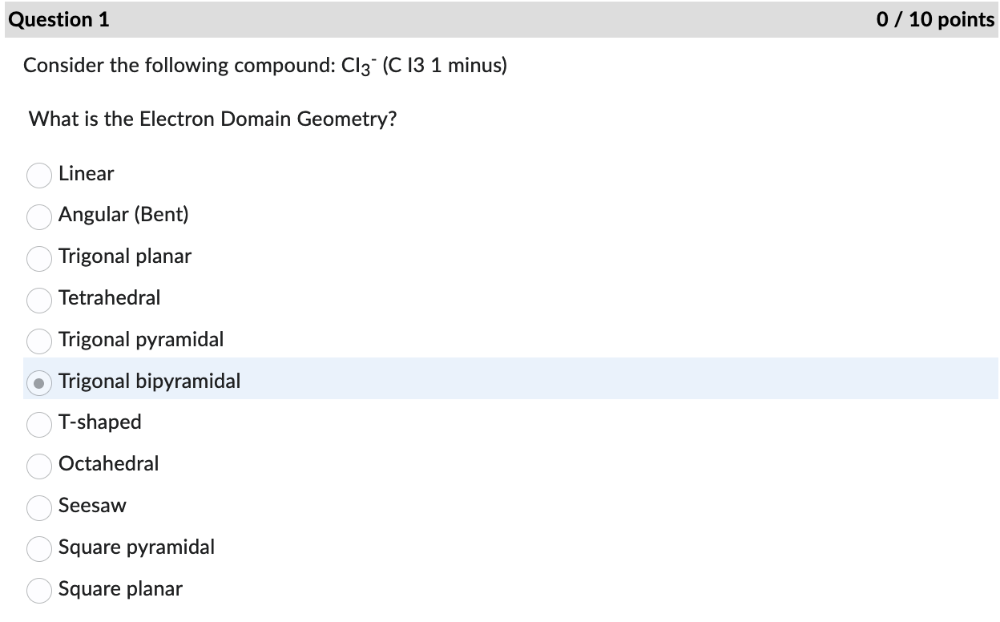
Trigonal bipyramidal
Consider the following compound: CH3 - (CH3 1 minus)
What is the Electron Domain Geometry?
Tetrahedral
Consider the following compound: NBr2 - (OR N Br2 1 minus)
What is the Electron Domain Geometry?
Tetrahedral
Consider the following compound: SbCl5
What is the Electron Domain Geometry?
Trigonal bipyramidal
Consider the following compound: S e Cl4
What is the Molecular Geometry?
Seesaw
Consider the following compound: AlCl3 2- (OR A l Cl3 2 minus)
What is the Molecular Geometry?
Trigonal pyramidal
Consider the following compound: T e F4
What is the Molecular Geometry?
Seesaw
Consider the following compound: S F4
What is the Molecular Geometry?
Seesaw
What is the hybridization of the central atom in NCl3 2- (OR N Cl3 2 minus)?
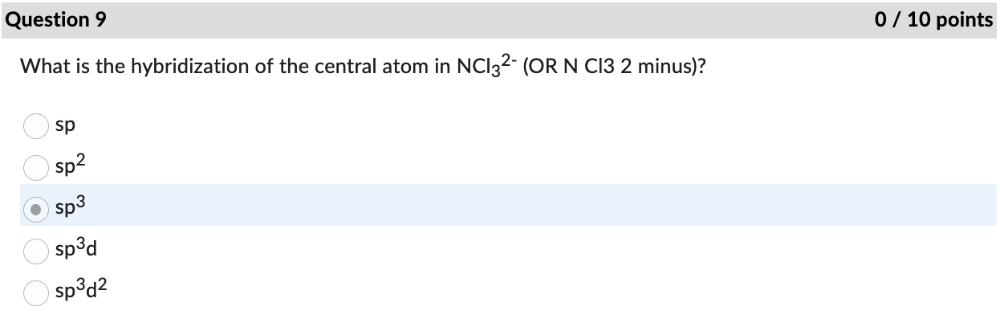
sp3
What is the hybridization of the central atom in AsCl2 - (OR A s Cl2 minus)?
sp3
What is the hybridization of the central atom in SbI2 - (OR S b I2 minus)?
sp3
What is the hybridization of the central atom in C F3 - (OR C F3 1 minus)?
sp3
What is the bond angle of the central atom in P I5?
120 o/ 90 o
What is the bond angle of the central atom in AsO2 - (OR AsO2 1 minus)?
120 o
What is the bond angle of the central atom in T e F4?
120 o/ 90 o
What is the bond angle of the central atom in S e F4?
120 o/ 90 o
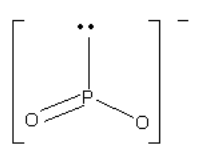
Consider the compound below:
Screen reader Note: P with lone pair straight up, in plane. O single bond in plane down to the right. O double bond down to the left. Bond angles appear to be 120 degrees. End of note.
Which arrow depicted below is the most accurate representation of the net dipole for this compound?
Consider the compound below:
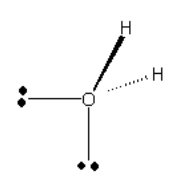
Screen Reader Note: O with lone pairs in plane. One points left, one points straight down. There are 2 "H" atoms. One points into the page up and to the right. One points out of the page up and to the right. End of note.
Which arrow depicted below is the most accurate representation of the net dipole for this compound?
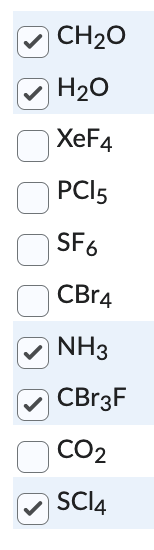
Which of the following molecules do you expect to be polar? You may need to draw the molecular geometries of each of the molecules to make this determination.
Select all correct answers.
Draw the Lewis Structure of C2H4.
C2H4 Contains how many total sigma bonds? (type a number (1,2,4, etc.)
______
Draw the Lewis Structure of C2H4.
C2H4 Contains how many total pi bonds? (type a number (1,2,4, etc.)
______
sigma = ___5___
pi = ___1___
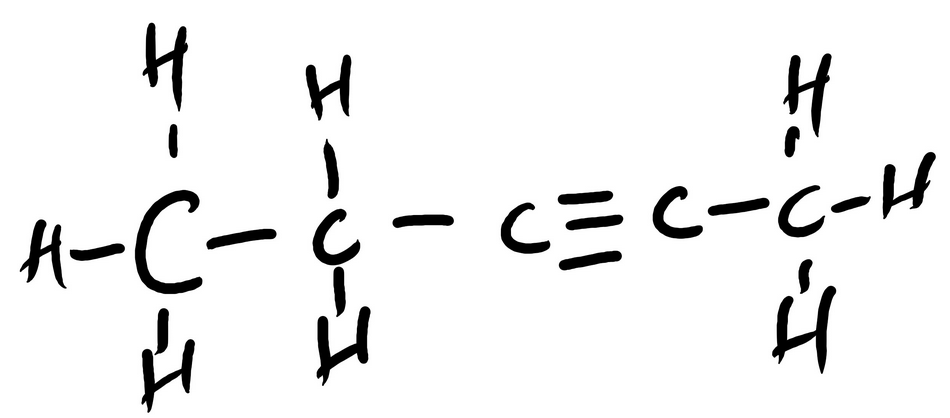
CH3CH2CCCH3 Contains how many total sigma bonds? (type a number (1,2,4, etc.)
______
CH3CH2CCCH3 Contains how many total pi bonds? (type a number (1,2,4, etc.)
______
sigma = ___12___
pi = ___2___
How many electrons are in bonding orbitals in O2?Be sure to use all the electrons when constructing your MO diagram. This includes the core and valence electrons.
10
How many electrons are in anti-bonding orbitals in B e2?Be sure to use all the electrons when constructing your MO diagram. This includes the core and valence electrons.
4
How many electrons are in bonding orbitals in B e2?
Be sure to use all the electrons when constructing your MO diagram. This includes the core and valence electrons.
4
How many electrons are in bonding orbitals in C2?Be sure to use all the electrons when constructing your MO diagram. This includes the core and valence electrons.
8
Draw a molecular orbital diagram for N2 -1. Is this molecule paramagnetic or diamagnetic?
- paramagnetic
- diamagnetic
paramagnetic